Hexadecacarbonylhexarhodium
| |
| Names | |
|---|---|
| IUPAC name
Hexadecacarbonylhexarhodium
| |
| Other names
Hexarhodium hexadecacarbonyl
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.044.539 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16O16Rh6 | |
| Molar mass | 1065.62 g/mol |
| Appearance | purple-brown solid |
| Melting point | 235 °C (455 °F; 508 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H312, H332 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
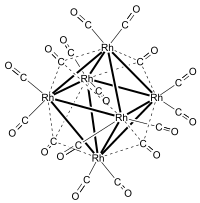
Hexadecacarbonylhexarhodium is a metal carbonyl cluster with the formula Rh6(CO)16. It exists as purple-brown crystals that are slightly soluble in dichloromethane and chloroform.[1] It is the principal binary carbonyl of rhodium.
Discovery and synthesis
Rh6(CO)16 was first prepared by Heiber in 1943 by carbonylation of RhCl3·3H2O at 80-230 °C and 200 atm carbon monoxide with silver or copper as a halide acceptor. Hieber correctly formulated the compound as a binary carbonyl, but suggested the formula Rh4(CO)11, i.e., CO/Rh ratio of 2.75.[2] The correct formula and structure was subsequently established by Dahl et al. using X-ray crystallography. The correct CO/Rh ratio is 2.66.[3]
Relative to the original preparation, the carbonylation of a mixture of anhydrous rhodium trichloride and iron pentacarbonyl was shown to give good yields of Rh6(CO)16.[4] Other compounds of rhodium are also effective precursors such as [(CO)2Rh(μ-Cl)]2 and rhodium(II) acetate:[1]
- 3 Rh2(O2CCH3)4 + 22 CO + 6 H2O → Rh6(CO)16 + 6 CO2 + 12 CH3COOH
- 3 [(CO)2RhCl]2 + 4 CO + 6 Cu → Rh6(CO)16 + 6 CuCl
Rh6(CO)16 catalyzes a number of organic reactions including hydrogenation and hydroformylation.[4]
References
- ^ a b James, B. R.; Rempel, G. L.; Teo, W. K. (1976). Hexadecacarbonylhexarhodium. Inorganic Syntheses. Vol. 16. p. 49. doi:10.1002/9780470132470.ch15. ISBN 9780470132470.
{{cite book}}:|journal=ignored (help) - ^ Hieber, W.; Lagally, H. (1943). "Über Metallcarbonyle. XLV. Das Rhodium im System der Metallcarbonyle". Zeitschrift für Anorganische und Allgemeine Chemie. 251 (1): 96–113. doi:10.1002/zaac.19432510110.
- ^ Corey, Eugene R.; Dahl, Lawrence F.; Beck, Wolfgang (1963). "Rh6(CO)16 and its Identity with Previously Reported Rh4(CO)11". J. Am. Chem. Soc. 85 (8): 1202–1203. doi:10.1021/ja00891a040.
- ^ a b Booth, B. L.; Else, M. J.; Fields, R.; Goldwhite, H.; Haszeldine, R. N. (1968). "Metal carbonyl chemistry IV. The preparation of cobalt and rhodium carbonyls by reductive carbonylation with pentacarbonyliron". J. Organomet. Chem. 14 (2): 417–422. doi:10.1016/S0022-328X(00)87682-2.
