Virome
Virome refers to the assemblage of viruses [1][2] that is often investigated and described by metagenomic sequencing of viral nucleic acids [3] that are found associated with a particular ecosystem, organism or holobiont. The word is frequently used to describe environmental viral shotgun metagenomes. Viruses, including bacteriophages, are found in all environments and studies of the virome have provided insights into nutrient cycling,[4] [5] development of immunity,[6] and a major source of genes through lysogenic conversion.[7]
History
The first comprehensive studies of viromes were by shotgun community sequencing,[8] which is frequently referred to as metagenomics. In the 2000s, the Rohwer lab sequenced viromes from seawater,[8][9] marine sediments,[10] adult human stool,[11] infant human stool,[12] soil,[13] and blood.[14] This group also performed the first RNA virome with collaborators from the Genomic Institute of Singapore.[15] From these early works, it was concluded that most of the genomic diversity is contained in the global virome and that most of this diversity remains uncharacterized.[16] This view was supported by individual genomic sequencing project, particularly the mycobacterium phage.[17]
Methods of study
In order to study the virome, virus-like particles are separated from cellular components, usually using a combination of filtration, density centrifugation, and enzymatic treatments to get rid of free nucleic acids.[18] The nucleic acids are then sequenced and analyzed using metagenomic methods. Alternatively, there are recent computational methods that use directly metagenomic assembled sequences to discover viruses.[19]
The Global Ocean Viromes (GOV) is a dataset consisting of deep sequencing from over 150 samples collected across the world's oceans in two survey periods by an international team.[20]
Virus hosts

Viruses are the most abundant biological entities on Earth, but challenges in detecting, isolating, and classifying unknown viruses have prevented exhaustive surveys of the global virome.[21] Over 5 Tb of metagenomic sequence data were used from 3,042 geographically diverse samples to assess the global distribution, phylogenetic diversity, and host specificity of viruses.[21]

In August 2016, over 125,000 partial DNA viral genomes, including the largest phage yet identified, increased the number of known viral genes by 16-fold.[21] A suite of computational methods was used to identify putative host virus connections.[21] The isolate viral host information was projected onto a group, resulting in host assignments for 2.4% of viral groups.[21]
Then the CRISPR–Cas prokaryotic immune system which holds a "library" of genome fragments from phages (proto-spacers) that have previously infected the host.[21] Spacers from isolate microbial genomes with matches to metagenomic viral contigs (mVCs) were identified for 4.4% of the viral groups and 1.7% of singletons.[21] The hypothesis was explored that viral transfer RNA (tRNA) genes originate from their host.[21]
Viral tRNAs identified in 7.6% of the mVCs were matched to isolate genomes from a single species or genus.[21] The specificity of tRNA-based host viral assignment was confirmed by CRISPR–Cas spacer matches showing a 94% agreement at the genus level. These approaches identified 9,992 putative host–virus associations enabling host assignment to 7.7% of mVCs.[21] The majority of these connections were previously unknown, and include hosts from 16 prokaryotic phyla for which no viruses have previously been identified.[21]
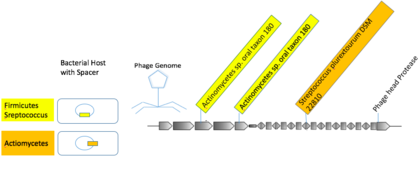
Many viruses specialize in infecting related hosts.[21] Viral generalists that infect hosts across taxonomic orders may exist.[21] Most CRISPR spacer matches were from viral sequences to hosts within one species or genus.[21] Some mVCs were linked to multiple hosts from higher taxa. A viral group composed of macs from human oral samples contained three distinct photo-spacers with nearly exact matches to spacers in Actionbacteria and Firmicutes.[21]
In January 2017, the IMG/VR system [22] -the largest interactive public virus database contained 265,000 metagenomic viral sequences and isolate viruses. This number scaled up to over 760,000 in November 2018 (IMG/VR v.2.0).[23] The IMG/VR systems serve as a starting point for the sequence analysis of viral fragments derived from metagenomic samples.
References
- ^ Anderson, Norman G; Gerin, John L; Anderson, N Leigh (2003). "Global Screening for Human Viral Pathogens". Emerging Infectious Diseases. 9 (7): 768–773. doi:10.3201/eid0907.030004. PMC 3023425. PMID 12890315.
- ^ Zárate, S; Taboada, B; Yocupicio-Monroy, M; Arias, CF (2017). "Human Virome". Archives of Medical Research. 48 (8): 701–716. doi:10.1016/j.arcmed.2018.01.005.
- ^ McDaniel, L; Breitbart, M; Mobberley, J; Long, A; Haynes, M; Rohwer, F; Paul, JH (23 September 2008). "Metagenomic analysis of lysogeny in Tampa Bay: implications for prophage gene expression". PLOS ONE. 3 (9): e3263. doi:10.1371/journal.pone.0003263. PMC 2533394. PMID 18810270.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Wilhelm, Steven W.; Suttle, Curtis A. (1999). "Viruses and Nutrient Cycles in the Sea". BioScience. 49 (10): 781–788. doi:10.2307/1313569. ISSN 1525-3244. JSTOR 1313569.
- ^ Wegley, L; Edwards, R; Rodriguez-Brito, B; Liu, H; Rohwer, F (November 2007). "Metagenomic analysis of the microbial community associated with the coral Porites astreoides". Environmental Microbiology. 9 (11): 2707–19. doi:10.1111/j.1462-2920.2007.01383.x. PMID 17922755.
- ^ Barr, JJ; Auro, R; Furlan, M; Whiteson, KL; Erb, ML; Pogliano, J; Stotland, A; Wolkowicz, R; Cutting, AS; Doran, KS; Salamon, P; Youle, M; Rohwer, F (25 June 2013). "Bacteriophage adhering to mucus provide a non-host-derived immunity". Proceedings of the National Academy of Sciences of the United States of America. 110 (26): 10771–6. doi:10.1073/pnas.1305923110. PMC 3696810. PMID 23690590.
- ^ Sharon, I; Battchikova, N; Aro, EM; Giglione, C; Meinnel, T; Glaser, F; Pinter, RY; Breitbart, M; Rohwer, F; Béjà, O (July 2011). "Comparative metagenomics of microbial traits within oceanic viral communities". The ISME Journal. 5 (7): 1178–90. doi:10.1038/ismej.2011.2. PMC 3146289. PMID 21307954.
- ^ a b Breitbart, M; Salamon, P; Andresen, B; Mahaffy, JM; Segall, AM; Mead, D; Azam, F; Rohwer, F (29 October 2002). "Genomic analysis of uncultured marine viral communities". Proceedings of the National Academy of Sciences of the United States of America. 99 (22): 14250–5. doi:10.1073/pnas.202488399. PMC 137870. PMID 12384570.
- ^ Angly, FE; Felts, B; Breitbart, M; Salamon, P; Edwards, RA; Carlson, C; Chan, AM; Haynes, M; Kelley, S; Liu, H; Mahaffy, JM; Mueller, JE; Nulton, J; Olson, R; Parsons, R; Rayhawk, S; Suttle, CA; Rohwer, F (November 2006). "The marine viromes of four oceanic regions". PLOS Biology. 4 (11): e368. doi:10.1371/journal.pbio.0040368. PMC 1634881. PMID 17090214.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Breitbart, M; Felts, B; Kelley, S; Mahaffy, JM; Nulton, J; Salamon, P; Rohwer, F (22 March 2004). "Diversity and population structure of a near-shore marine-sediment viral community". Proceedings of the Royal Society B: Biological Sciences. 271 (1539): 565–74. doi:10.1098/rspb.2003.2628. PMC 1691639. PMID 15156913.
- ^ Breitbart, M; Hewson, I; Felts, B; Mahaffy, JM; Nulton, J; Salamon, P; Rohwer, F (October 2003). "Metagenomic analyses of an uncultured viral community from human feces". Journal of Bacteriology. 185 (20): 6220–3. doi:10.1128/jb.185.20.6220-6223.2003. PMC 225035. PMID 14526037.
- ^ Breitbart, M; Haynes, M; Kelley, S; Angly, F; Edwards, RA; Felts, B; Mahaffy, JM; Mueller, J; Nulton, J; Rayhawk, S; Rodriguez-Brito, B; Salamon, P; Rohwer, F (June 2008). "Viral diversity and dynamics in an infant gut". Research in Microbiology. 159 (5): 367–73. doi:10.1016/j.resmic.2008.04.006. PMID 18541415.
- ^ Fierer, N; Breitbart, M; Nulton, J; Salamon, P; Lozupone, C; Jones, R; Robeson, M; Edwards, RA; Felts, B; Rayhawk, S; Knight, R; Rohwer, F; Jackson, RB (November 2007). "Metagenomic and small-subunit rRNA analyses reveal the genetic diversity of bacteria, archaea, fungi, and viruses in soil". Applied and Environmental Microbiology. 73 (21): 7059–66. doi:10.1128/aem.00358-07. PMC 2074941. PMID 17827313.
- ^ Breitbart, M; Rohwer, F (November 2005). "Method for discovering novel DNA viruses in blood using viral particle selection and shotgun sequencing". BioTechniques. 39 (5): 729–36. doi:10.2144/000112019. PMID 16312220.
- ^ Zhang, T; Breitbart, M; Lee, WH; Run, JQ; Wei, CL; Soh, SW; Hibberd, ML; Liu, ET; Rohwer, F; Ruan, Y (January 2006). "RNA viral community in human feces: prevalence of plant pathogenic viruses". PLOS Biology. 4 (1): e3. doi:10.1371/journal.pbio.0040003. PMC 1310650. PMID 16336043.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Edwards, RA; Rohwer, F (June 2005). "Viral metagenomics". Nature Reviews. Microbiology. 3 (6): 504–10. doi:10.1038/nrmicro1163. PMID 15886693.
- ^ Rohwer, F (18 April 2003). "Global phage diversity". Cell. 113 (2): 141. doi:10.1016/s0092-8674(03)00276-9. PMID 12705861.
- ^ Thurber, RV; Haynes, M; Breitbart, M; Wegley, L; Rohwer, F (2009). "Laboratory procedures to generate viral metagenomes". Nature Protocols. 4 (4): 470–83. doi:10.1038/nprot.2009.10. PMID 19300441.
- ^ Paez-Espino D, Pavlopoulos GA, Ivanova NN, Kyrpides NC (August 2017). "Nontargeted virus sequence discovery pipeline and virus clustering for metagenomic data". Nat Protoc. 12 (8): 1673–1682. doi:10.1038/nprot.2017.063. PMID 28749930.
- ^ Tang, Lei (July 2019). "Pole-to-pole ocean viromes". Research highlights. Nature Methods (Paper). 16: 575. doi:10.1038/s41592-019-0480-1. – via Springer Nature (subscription required)
- ^ a b c d e f g h i j k l m n o Paez-Espino D, Eloe-Fadrosh EA, Pavlopoulos GA, Thomas AD, Huntemann M, Mikhailova N, Rubin E, Ivanova NN, Kyrpides NC (August 2016). "Uncovering Earth's virome". Nature. 536 (7617): 425–30. doi:10.1038/nature19094. PMID 27533034.
- ^ Paez-Espino D, Chen IA, Palaniappan K, Ratner A, Chu K, Szeto E, et al. (January 2017). "IMG/VR: a database of cultured and uncultured DNA Viruses and retroviruses". Nucleic Acids Res. 45 (D1): D457–D465. doi:10.1093/nar/gkw1030. PMC 5210529. PMID 27799466.
- ^ Paez-Espino D, Roux S, Chen IA, Palaniappan K, Ratner A, Chu K, et al. (2019). "IMG/VR v.2.0: an integrated data management and analysis system for cultivated and environmental viral genomes". Nucleic Acids Res. 47 (Database issue): D678–D686. doi:10.1093/nar/gky1127. PMC 6323928. PMID 30407573.
