2-Methylimidazole

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methyl-1H-imidazole | |
| Other names
2-MeIm
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.010.697 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6N2 | |
| Molar mass | 82.10 g/mol |
| Appearance | white or colorless solid |
| Melting point | 145 °C (293 °F; 418 K) |
| Boiling point | 270 °C (518 °F; 543 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
causes skin rashes and eye irritation |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Methylimidazole is an organic compound that is structurally related to imidazole with the chemical formula CH3C3H2N2H. It is a white or colorless solid that is highly soluble in polar organic solvents and water. It is a precursor to a range of drugs and is a ligand in coordination chemistry.
Synthesis and reactions
It is prepared by condensation of glyoxal, ammonia, and acetaldehyde, a Radziszewski reaction. Nitration gives 5-nitro derivative.[1]
2-Methylimidazole is a sterically hindered imidazole that is used to simulate the coordination of histidine to heme complexes. It can be deprotonated to make imidazolate-based coordination polymers.[2]
Applications
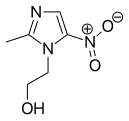
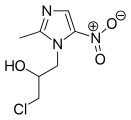
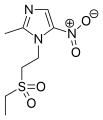
2-Methylimidazole is a precursor to the several members of the nitroimidazole antibiotics that are used to combat anaerobic bacterial and parasitic infections.[3][1]
- Nitroimidazole antibiotics and antiprotozoals containing 2-methylimidazole cores:
Safety
It has low toxicity with an LD50 (rat, oral) of 1300 mg/kg, but it is strongly irritating to the skin and eyes.[1]
References
- ^ a b c Ebel, K., Koehler, H., Gamer, A. O., & Jäckh, R. "Imidazole and Derivatives." In Ullmann’s Encyclopedia of Industrial Chemistry; 2002 Wiley-VCH, doi:10.1002/14356007.a13_661
- ^ Banerjee, Rahul; Phan, Anh; Wang, Bo; Knobler, Carolyn; Furukawa, Hiroyasu; O'Keeffe, Michael; Yaghi, Omar M (2008). "High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture". Science. 319: 939–943. doi:10.1126/science.1152516. PMID 18276887.
- ^ Edwards, David I (1993). "Nitroimidazole drugs - action and resistance mechanisms. I. Mechanism of action". Journal of Antimicrobial Chemotherapy. 31: 9–20. doi:10.1093/jac/31.1.9.