Patchoulol

| |

| |
| Names | |
|---|---|
| IUPAC name
3,4,4αβ,5,6β,7,8,8α-Octahydro-4α,8αβ,9,9-
| |
| Other names
Patchouli camphor;
(–)-patchoulol; (1R,3R,6S,7S,8S)-patchoulol; patchouli alcohol | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.025.279 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H26O | |
| Molar mass | 222.36 |
| Appearance | Hexagonal-trapezohedral crystals |
| Density | 1.0284 g/mL |
| Melting point | 56 °C (133 °F; 329 K) (racemic) |
| Boiling point | 287–288 °C (549–550 °F; 560–561 K) |
| practically insoluble | |
| Solubility in ethanol | soluble |
| Solubility in diethyl ether | soluble |
Refractive index (nD)
|
1.5029 |
| Hazards | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Patchoulol or patchouli alcohol (C15H26O) is a sesquiterpene alcohol found in patchouli.[1] Patchouli oil is an important material in perfumery. The (−)-optical isomer is one of the organic compounds responsible for the typical patchouli scent. Patchoulol is also used in the synthesis of the chemotherapy drug Taxol.
Structure determination
Patchouli alcohol was first isolated in 1869 by Gal and its chemical composition later correctly formulated as C15H26O by Montgolfier.[2] During early structural investigation the presence of a saturated tricyclic tertiary alcohol was established.[3] After several years of careful study Büchi and co-workers proposed the structure of patchouli alcohol to correspond to 1, based on degradation studies from his earlier work, verified later by synthesis of material which corresponded to the natural authentic sample of patchouli alcohol.[4]
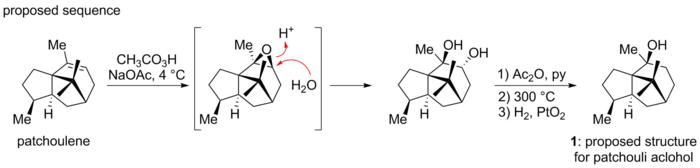
A serendipitous finding by Dunitz and co-workers revealed a contradictory structure. They had undertaken an X-ray analysis of the patchouli alcohol diester of chromic acid, with the objective of determining the Cr-O-C angles. In the course of their analysis they found that the X-ray evidence could not be reconciled with the proposed structure 1.[5] Instead they proposed together with Büchi the novel structure 2. The discrepancy had resulted from an unanticipated skeletal rearrangement that had occurred in the Büchi synthesis when patchoulene was treated with peroxy acid, an operation that by accident generated the correct architecture of the natural product.[6]

Contains embedded [2.2.2]propellane motif.
See also
References
- ^ Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. (2006). "The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases". Archives of Biochemistry and Biophysics. 454 (2): 123–136. doi:10.1016/j.abb.2006.08.006. PMID 16970904.
- ^ Büchi, G.; Erickson, R. E.; Wakabyashi, N. (1961). "Terpenes. XVI. Constitution of Patchouli Alcohol and Absolute Configuration of Cedrene". Journal of the American Chemical Society. 83: 927. doi:10.1021/ja01465a042.
- ^ Simonsen, J.; Barton, D. H. R. (1952). The Terpenes. Vol. Vol. 111. Cambridge University Press, London.
{{cite book}}:|volume=has extra text (help) - ^ Büchi, G.; Macleod, W. D. (1962). "Synthesis of Patchouli Alcohol". Journal of the American Chemical Society. 84: 3205–3206. doi:10.1021/ja00875a047.
- ^ Dobler, M.; Dunitz, J. D.; Gubler, B., Weber, H. P.; Büchi, G.; Padilla, O. J. (1963). "The Structure of Patchouli Alcohol". Proc. Chem. Soc. December: 383. doi:10.1039/PS9630000357.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Nicolaou, K. C.; Snyder, S. A. (2005). "Chasing Molecules That Were Never There: Misassigned Natural Products and the Role of Chemical Synthesis in Modern Structure Elucidation". Angewandte Chemie International Edition. 44: 1012–1044. doi:10.1002/anie.200460864. PMID 15688428.
