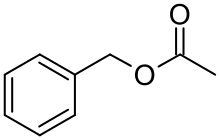
Benzyl acetate
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Benzyl acetate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.909 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H10O2 | |
| Molar mass | 150.18 g/mol |
| Appearance | Colourless liquid |
| Odor | flowery |
| Density | 1.054 g/ml |
| Melting point | −51.5 °C (−60.7 °F; 221.7 K) |
| Boiling point | 212 °C (414 °F; 485 K) |
| 0.31 g/100 mL | |
| Solubility | Soluble in benzene, chloroform Miscible with ethanol, ether, acetone |
| -93.18·10−6 cm3/mol | |
Refractive index (nD)
|
1.523 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 102 °C (216 °F; 375 K) |
| 461 °C (862 °F; 734 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzyl acetate is an organic ester with the molecular formula C9H10O2. It is formed by the condensation of benzyl alcohol and acetic acid.
Similar to most other esters, it possesses a sweet and pleasant aroma, owing to which, it finds applications in personal hygiene and health care products. It is a constituent of jasmin and of the essential oils of ylang-ylang and neroli.[1] It has pleasant sweet aroma reminiscent of jasmine. Further as a flavoring agent it is also used to impart jasmine or apple flavors to various cosmetics and personal care products like lotions, hair creams etc..[2]
It is one of many compounds that is attractive to males of various species of orchid bees. It is collected and used by the bees as an intra-specific pheromone; In apiculture benzyl acetate is used as a bait to collect bees. Natural sources of benzyl acetate include varieties of flowers like jasmine (Jasminum), and fruits like pear, apple, etc.[3]
Industrially benzyl acetate is used as a medium of extraction in extraction of plastics, resin, cellulose acetate, cellulose nitrate, oils and lacquers.[citation needed]
References
- ^ "Benzyl Acetate - an overview | ScienceDirect Topics".
- ^ "Benzyl acetate". The Good Scents Company.
- ^ Schiestl, F.P.; Roubik, D.W. (2004). "Odor Compound Detection in Male Euglossine Bees". Journal of Chemical Ecology. 29 (1): 253–257. doi:10.1023/A:1021932131526. PMID 12647866.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help)

