Large for gestational age
| Large for gestational age | |
|---|---|
| Other names | Macrosomia |
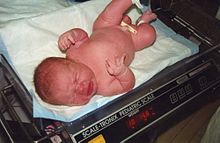 | |
| LGA: A healthy 11-pound (5.0 kg) newborn child, delivered vaginally without complications (41 weeks; fourth child; no gestational diabetes) | |
| Specialty | Obstetrics, pediatrics |
Large for gestational age (LGA) describes full-term or post-term infants that are born of high birth weight.[1]
The term LGA or large for gestational age is defined by birth weight above the 90th percentile for their gestational age and gender.[2] In infants with birth weight above the 97th percentile in their gestational age and gender group, research has shown that greater risk of long-term health complications and fatal outcomes are present in LGA infants.[3]
Specifically, large for gestational age can be characterized by macrosomia, referring to a fetal growth beyond a certain threshold (threshold ranging from a body weight of 4,000 grams to above 5,000 grams).[4] Experts in Obstetrics and Gynecology currently use a grading system to evaluate LGA infants, where their birth weight may help identify risks associated with their birth, including labor complications of both mother and child, potential long-term health complications of the neonate and infant mortality.[3]
Presentation
Complications
Neonatal
Common risks in LGA babies include shoulder dystocia,[3] hypoglycemia,[3] brachial plexus injuries,[5] metatarsus adductus, hip subluxation [6] and talipes calcaneovalgus, due to intrauterine deformation.[6]
Shoulder dystocia occurs when the anterior shoulder becomes impacted on the maternal pubic symphysis during birth.[7] The doctor or midwife will try to push the baby's anterior shoulder downward to pass through the birth canal and clear the woman's pubic symphysis. This can be difficult if the child is LGA, since the birth canal is 10 cm when fully dilated for most women and there may not be much room to move the baby. If shoulder dystocia occurs, there are various maneuvers which can be performed by the birth attendant to try to deliver the shoulders. These generally involve trying to turn the shoulders into the oblique, using suprapubic pressure to disimpact the anterior shoulder from above the pubic symphysis, or delivering the posterior arm first.[8] If these do not resolve the situation, the provider may intentionally snap the baby's clavicle (bone that holds shoulder in place) in a procedure called cleidotomy in order to displace the shoulder and allow the child to be delivered.[9][10] Other methods to deliver the baby as a last resort when all else have failed are the Zavanelli maneuver and symphysiotomy.[9][10] The Zavanelli maneuver involves flexing and pushing the fetal head back into the birth canal, and an emergency cesarean section is then performed.[10] Symphysiotomy allows childbirth by surgically dividing the pubic bone to widen the pelvis and it is performed after a failed Zavanelli maneuver.[10] Newborns with shoulder dystocia are at risk of temporary or permanent nerve damage to the baby's arm, or other injuries such as humeral fracture.[8]
In non-diabetic women, shoulder dystocia happens 0.65% of the time in babies that weigh less than 8 pounds 13 ounces (4,000 g), 6.7% of the time in babies that weigh 8 pounds 13 ounces (4,000 g) to 9 pounds 15 ounces (4,500 g), and 14.5% of the time in babies that weigh more than 9 pounds 15 ounces (4,500 g).[11] In diabetic women, shoulder dystocia happens 2.2% of the time in babies that weigh less than 8 pounds 13 ounces (4,000 g), 13.9% of the time in babies that weigh 8 pounds 13 ounces (4,000 g) to 9 pounds 15 ounces (4,500 g), and 52.5% of the time in babies that weigh more than 9 pounds 15 ounces (4,500 g).[11] Although big babies are at higher risk for shoulder dystocia, most cases of shoulder dystocia happen in smaller babies because there are many more small and normal-size babies being born than big babies.[12] Researchers have been unable to predict who will have shoulder dystocia and who will not.[13]
LGA babies are at higher risk of hypoglycemia in the neonatal period, independent of whether the mother has diabetes.[14] Hypoglycemia, as well as hyperbilirubinemia and polycythemia, occur as a result of hyperinsulinemia in the fetus.[15]
High birth weight may impact the baby in the long term. Macrosomic neonates are at a higher risk of being overweight and obese than their normal-weight counterparts later in life.[4][16] Studies have shown that the long-term overweight risk is doubled when the birth weight is greater than 4,000 g.[17] The risk of having type 2 diabetes mellitus in adult life is 19% higher among macrosomic babies with birth weights heavier than 4,500 g compared to those with birth weights between 4,000 g and 4,500 g.[18]
Maternal
Maternal complications in pregnancies with macrosomia include emergency cesarean section, postpartum hemorrhage and obstetric anal sphincter injury.[19] The risk of maternal complications in pregnancies with newborns weighing between 4,000 g and 4,500 g is two-fold greater than in pregnancies without macrosomia. In pregnancies with newborns weighing over 4,500 g, the risk is approximately three-fold greater.[19]
Risk factors
Maternal diabetes
One of the primary risk factors of LGA is poorly-controlled maternal diabetes, particularly gestational diabetes (GD), as well as preexisting diabetes mellitus (DM) (preexisting type 2 is associated more with macrosomia, while preexisting type 1 can be associated with microsomia).[20] The risk of having a macrosomic fetus is three times greater in mothers with diabetes than those without diabetes.[21] DM increases maternal plasma glucose levels as well as insulin, stimulating fetal growth of subcutaneous fat.[21] The LGA newborn exposed to maternal DM usually only has an increase in weight, not a change in body length or head size.[21]
Genetics
Genetics plays a role in having an LGA baby. Taller, heavier parents tend to have larger babies.[22] Genetic disorders of overgrowth (e.g. Beckwith–Wiedemann syndrome, Sotos syndrome, Perlman syndrome, Simpson-Golabi-Behmel syndrome [23]) are often characterized by macrosomia.[24][25]
Other risk factors
- Gestational age: pregnancies that go beyond 40 weeks increase incidence of an LGA infant [21]
- Fetal sex: male infants tend to weigh more than female infants [5]
- Obesity prior to pregnancy and maternal weight gain above recommended guidelines during pregnancy [26][27][28]
- Multiparity: giving birth to previous LGA infants vs. non-LGA infants [5]
- Frozen embryo transfer as fertility treatment, as compared with fresh embryo transfer or no artificial assistance [29][30]
Diagnosis

Diagnosing fetal macrosomia cannot be performed until after birth, as evaluating a baby's weight in the womb may be inaccurate.[21] While ultrasound has been the primary method for diagnosing LGA, this form of fetal weight assessment remains imprecise, as the fetus is a highly variable structure in regards to density and weight— no matter the gestational age.[21] Ultrasonography involves an algorithm that incorporates biometric measurements of the fetus, such as biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), and femur length (FL), to calculate the estimated fetal weight (EFW).[31] Variability of fetal weight estimations has been linked to differences due to sensitivity and specificity of ultrasound algorithms as well as to the individual performing the ultrasound examination.[32]
In addition to sonography, fetal weight can also be assessed using clinical and maternal methods. Clinical methods for estimating fetal weight involves measuring the mother's symphysis-fundal height and performing Leopold's maneuvers, which can help with determining the fetus position in utero in addition to size.[32] However, as this method relies heavily on practitioner experience and technique, it does not provide an accurate and definite diagnosis of an LGA infant and only would only serve as a potential indication of suspected macrosomia.[32] Fetal weight can also be estimated through a mother's subjective assessment of the fetus size, but this method is dependent on a mother's experience with past pregnancies and may not be clinically useful.[32] There are new methods being studied for their accuracy in predicting fetal weight, such as measuring fetal soft tissue, but more research needs to be done to find a consistent, reliable method.[33]
Management
Induction of labor at or near term for women with a baby of suspected macrosomia has been proposed as a treatment method, as it stops fetal growth and results in babies with a lower birth weight, fewer bone fractures, and less incidence of shoulder dystocia.[7] However, this method could increase the number of women with perineal tears, and failed inductions can prompt the need for emergency cesarean sections.[7] LGA babies are more than two times likely to be delivered by Cesarean section, compared to infants under 4000 grams (below the threshold of macrosomia).[34] Predicting a baby’s weight can be inaccurate and women could be worried unnecessarily, and request their labor to be induced without a medical reason.[7] Doctors disagree whether women should be induced for suspected macrosomia and more research is needed to find out what is best for women and their babies.[7]
Elective cesarean section has also been presented as a potential delivery method for infants of suspected macrosomia, as it can serve to prevent possible birth trauma. However, the American College of Obstetricians and Gynecologists recommends that cesarean delivery should only be considered if the fetus is an estimated weight of at least 5,000 grams in non-diabetic mothers and at least 4,500 grams in diabetic mothers. [35] A number needed to treat analysis determined that approximately 3,700 women with suspected fetal macrosomia would have to undergo an unnecessary cesarean section in order to prevent one incident of brachial plexus injuries secondary to shoulder dystocia.[5]
Management of gestational diabetes through dietary modifications and anti-diabetic medications has been shown to decrease the incidence of LGA.[36] The use of metformin to control maternal blood glucose levels has shown to be more effective than using insulin alone in reducing the likelihood of fetal macrosomia.[37] There is a 20% lower chance of having an LGA baby when using metformin to manage diabetes compared to using insulin.[38]
Modifiable risk factors that increase the incidence of LGA births, such as gestational weight gain above recommended BMI guidelines, can be managed with lifestyle modifications, including maintaining a balanced diet and exercising.[39][40] Such interventions can help mothers achieve the recommended gestational weight and lower the incidence of fetal macrosomia in obese and overweight women.[39][40] The World Health Organization also recommends that mothers aim for their recommended BMI prior to conception.[28] In general, obese mothers or women with excessive gestational weight gain may have higher risk of pregnancy complications (ranging from LGA, shoulder dystocia, etc.). [41]
Epidemiology
In healthy pregnancies without pre-term or post-term health complications, large for gestational age, or fetal macrosomia have been observed to affect around 12% of newborns. [7] By comparison, women with gestational diabetes are at an increased risk of giving birth to LGA babies, where ~15-45% of neonates may be affected.[7] In 2017, the National Center of Health Statistics found that 7.8% of live-born infants born in the United States meet the definition of macrosomia, where their birth weight surpasses the threshold of 4000 grams (above ~8.8 pounds).[7] Women in Europe and the United States tend to have higher pre-term body weight and have increased gestational weight during pregnancy compared to women in east Asia.[42] Thus, women in Europe and the United States, with higher gestational weight gain, tend to have higher associated risk of LGA infants, macrosomia and cesarean.[42] In European countries, the prevalence of births of newborns weighing between 4,000 g and 4,499 g is 8% to 21%, and in Asian countries the prevalence is between 1% and 8%.[43] In general, rates of LGA infants have increased 15-25% in many countries including the United States, Canada, Germany, Denmark, Scotland and more in the past 20-30 years, suggesting an increase in LGA births worldwide.[44]
References
- ^ Henriksen T (2008). "The macrosomic fetus: a challenge in current obstetrics". Acta Obstetricia et Gynecologica Scandinavica. 87 (2): 134–45. doi:10.1080/00016340801899289. PMID 18231880. S2CID 38118355.
- ^ McGrath RT, Glastras SJ, Hocking SL, Fulcher GR (August 2018). "Large-for-Gestational-Age Neonates in Type 1 Diabetes and Pregnancy: Contribution of Factors Beyond Hyperglycemia". Diabetes Care. 41 (8): 1821–1828. doi:10.2337/dc18-0551. PMID 30030258. S2CID 207369659.
- ^ a b c d Boulet SL, Alexander GR, Salihu HM, Pass M (May 2003). "Macrosomic births in the united states: determinants, outcomes, and proposed grades of risk". American Journal of Obstetrics and Gynecology. 188 (5): 1372–8. doi:10.1067/mob.2003.302. PMID 12748514.
- ^ a b Barth Jr WH, Jackson R, et al. (Committee on Practice Bulletins—Obstetrics) (January 2020). "Macrosomia: ACOG Practice Bulletin, Number 216". Obstetrics and Gynecology. 135 (1): e18–e35. doi:10.1097/AOG.0000000000003606. PMID 31856124.
- ^ a b c d Zamorski MA, Biggs WS (January 2001). "Management of suspected fetal macrosomia". American Family Physician. 63 (2): 302–6. PMID 11201695.
- ^ a b Lapunzina P, Camelo JS, Rittler M, Castilla EE (February 2002). "Risks of congenital anomalies in large for gestational age infants". The Journal of Pediatrics. 140 (2): 200–4. doi:10.1067/mpd.2002.121696. PMID 11865271.
- ^ a b c d e f g h Boulvain M, Irion O, Dowswell T, Thornton JG, et al. (Cochrane Pregnancy and Childbirth Group) (May 2016). "Induction of labour at or near term for suspected fetal macrosomia". The Cochrane Database of Systematic Reviews (5): CD000938. doi:10.1002/14651858.CD000938.pub2. PMC 7032677. PMID 27208913.
- ^ a b Young BC, Ecker JL (February 2013). "Fetal macrosomia and shoulder dystocia in women with gestational diabetes: risks amenable to treatment?". Current Diabetes Reports. 13 (1): 12–8. doi:10.1007/s11892-012-0338-8. PMID 23076441. S2CID 4385185.
- ^ a b Politi S, D'emidio L, Cignini P, Giorlandino M, Giorlandino C (July 2010). "Shoulder dystocia: an Evidence-Based approach". Journal of Prenatal Medicine. 4 (3): 35–42. PMC 3279180. PMID 22439059.
- ^ a b c d Hill MG, Cohen WR (2016). "Shoulder dystocia: prediction and management". Women's Health. 12 (2): 251–61. doi:10.2217/whe.15.103. PMC 5375046. PMID 26901875.
- ^ a b Rouse DJ, Owen J, Goldenberg RL, Cliver SP (November 1996). "The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound". JAMA. 276 (18): 1480–6. doi:10.1001/jama.1996.03540180036030. PMID 8903259.
- ^ Morrison JC, Sanders JR, Magann EF, Wiser WL (December 1992). "The diagnosis and management of dystocia of the shoulder". Surgery, Gynecology & Obstetrics. 175 (6): 515–22. PMID 1448731.
- ^ Gross TL, Sokol RJ, Williams T, Thompson K (June 1987). "Shoulder dystocia: a fetal-physician risk". American Journal of Obstetrics and Gynecology. 156 (6): 1408–18. doi:10.1016/0002-9378(87)90008-1. PMID 3591856.
- ^ Rozance PJ (February 2014). "Update on neonatal hypoglycemia". Current Opinion in Endocrinology, Diabetes and Obesity. 21 (1): 45–50. doi:10.1097/MED.0000000000000027. PMC 4012366. PMID 24275620.
- ^ Mayer C, Joseph KS (February 2013). "Fetal growth: a review of terms, concepts and issues relevant to obstetrics". Ultrasound in Obstetrics & Gynecology. 41 (2): 136–45. doi:10.1002/uog.11204. PMID 22648955.
- ^ Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C (October 2005). "Being big or growing fast: systematic review of size and growth in infancy and later obesity". BMJ. 331 (7522): 929. doi:10.1136/bmj.38586.411273.E0. PMC 1261184. PMID 16227306.
- ^ Schellong K, Schulz S, Harder T, Plagemann A (2012). Hernandez AV (ed.). "Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally". PLOS ONE. 7 (10): e47776. Bibcode:2012PLoSO...747776S. doi:10.1371/journal.pone.0047776. PMC 3474767. PMID 23082214.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Knop MR, Geng TT, Gorny AW, Ding R, Li C, Ley SH, Huang T (December 2018). "Birth Weight and Risk of Type 2 Diabetes Mellitus, Cardiovascular Disease, and Hypertension in Adults: A Meta-Analysis of 7 646 267 Participants From 135 Studies". Journal of the American Heart Association. 7 (23): e008870. doi:10.1161/JAHA.118.008870. PMC 6405546. PMID 30486715.
- ^ a b Beta J, Khan N, Khalil A, Fiolna M, Ramadan G, Akolekar R (September 2019). "Maternal and neonatal complications of fetal macrosomia: systematic review and meta-analysis". Ultrasound in Obstetrics & Gynecology. 54 (3): 308–318. doi:10.1002/uog.20279. PMID 30938004.
- ^ Leipold H, Worda C, Gruber CJ, Kautzky-Willer A, Husslein PW, Bancher-Todesca D (August 2005). "Large-for-gestational-age newborns in women with insulin-treated gestational diabetes under strict metabolic control". Wiener Klinische Wochenschrift. 117 (15–16): 521–5. doi:10.1007/s00508-005-0404-1. PMID 16160802. S2CID 7465313.
- ^ a b c d e f Kc K, Shakya S, Zhang H (2015). "Gestational diabetes mellitus and macrosomia: a literature review". Annals of Nutrition & Metabolism. 66 Suppl 2 (Suppl. 2): 14–20. doi:10.1159/000371628. PMID 26045324. S2CID 6536421.
- ^ Christian M, Gustafsson J. Pediatrik (2 uppl ed.). ISBN 9789147112968. OCLC 1001668564.
- ^ Sajorda BJ, Gonzalez-Gandolfi CX, Hathaway ER, Kalish JM (1993–2020). "Simpson-Golabi-Behmel Syndrome Type 1". GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle. PMID 20301398.
- ^ "Beckwith-Wiedemann syndrome". Genetics Home Reference. Retrieved 2020-07-30.
- ^ Vora N, Bianchi DW (October 2009). "Genetic considerations in the prenatal diagnosis of overgrowth syndromes". Prenatal Diagnosis. 29 (10): 923–9. doi:10.1002/pd.2319. PMC 4426974. PMID 19609940.
- ^ Boubred F, Pauly V, Romain F, Fond G, Boyer L (2020-06-05). Farrar D (ed.). "The role of neighbourhood socioeconomic status in large for gestational age". PLOS ONE. 15 (6): e0233416. Bibcode:2020PLoSO..1533416B. doi:10.1371/journal.pone.0233416. PMC 7274403. PMID 32502147.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Henriksen T (February 2008). "The macrosomic fetus: a challenge in current obstetrics". Acta Obstetricia et Gynecologica Scandinavica. 87 (2): 134–45. doi:10.1080/00016340801899289. PMID 18231880. S2CID 38118355.
- ^ a b Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. (June 2017). "Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis". JAMA. 317 (21): 2207–2225. doi:10.1001/jama.2017.3635. PMC 5815056. PMID 28586887.
- ^ Berntsen S, Pinborg A (May 2018). "Large for gestational age and macrosomia in singletons born after frozen/thawed embryo transfer (FET) in assisted reproductive technology (ART)". Birth Defects Research. 110 (8): 630–643. doi:10.1002/bdr2.1219. PMID 29714057. S2CID 25437950.
- ^ Orvieto R, Kirshenbaum M, Gleicher N (2020). "Is Embryo Cryopreservation Causing Macrosomia-and What Else?". Frontiers in Endocrinology. 11: 19. doi:10.3389/fendo.2020.00019. PMC 6997460. PMID 32047479.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Coomarasamy A, Connock M, Thornton J, Khan KS (November 2005). "Accuracy of ultrasound biometry in the prediction of macrosomia: a systematic quantitative review". BJOG. 112 (11): 1461–6. doi:10.1111/j.1471-0528.2005.00702.x. PMID 16225563. S2CID 34330598.
- ^ a b c d Ray EM, Alhusen JL (2016). "The Suspected Macrosomic Fetus at Term: A Clinical Dilemma". Journal of Midwifery & Women's Health. 61 (2): 263–9. doi:10.1111/jmwh.12414. PMID 26869131.
- ^ Warska A, Maliszewska A, Wnuk A, Szyszka B, Sawicki W, Cendrowski K (March 2018). "Current knowledge on the use of ultrasound measurements of fetal soft tissues for the assessment of pregnancy development". Journal of Ultrasonography. 18 (72): 50–55. doi:10.15557/JoU.2018.0008. PMC 5911719. PMID 29844941.
- ^ Steer P (February 2004). "The management of large and small for gestational age fetuses". Seminars in Perinatology. 28 (1): 59–66. doi:10.1053/j.semperi.2003.10.013. PMID 15058903.
- ^ "Safe Prevention of the Primary Cesarean Delivery". www.acog.org. Retrieved 2020-08-04.
- ^ Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L (July 2013). "Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research". Annals of Internal Medicine. 159 (2): 123–9. doi:10.7326/0003-4819-159-2-201307160-00661. PMID 23712381. S2CID 21881403.
- ^ Guo L, Ma J, Tang J, Hu D, Zhang W, Zhao X (2019). "Comparative Efficacy and Safety of Metformin, Glyburide, and Insulin in Treating Gestational Diabetes Mellitus: A Meta-Analysis". Journal of Diabetes Research. 2019: 9804708. doi:10.1155/2019/9804708. PMC 6875019. PMID 31781670.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Butalia S, Gutierrez L, Lodha A, Aitken E, Zakariasen A, Donovan L (January 2017). "Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: a systematic review and meta-analysis". Diabetic Medicine. 34 (1): 27–36. doi:10.1111/dme.13150. PMID 27150509. S2CID 3418227.
- ^ a b World Health Organization (2016). WHO recommendations on antenatal care for a positive pregnancy experience. Geneva, Switzerland. ISBN 978-92-4-154991-2. OCLC 974355266.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ a b "Weight Gain During Pregnancy | Pregnancy | Maternal and Infant Health C". www.cdc.gov. 2019-01-17. Retrieved 2020-08-04.
- ^ Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergström A, et al. (July 2019). "Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts". BJOG. 126 (8): 984–995. doi:10.1111/1471-0528.15661. PMC 6554069. PMID 30786138.
- ^ a b Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, et al. (August 2018). "Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women". BMC Medicine. 16 (1): 153. doi:10.1186/s12916-018-1128-1. PMC 6117916. PMID 30165842.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Culliney KA, Parry GK, Brown J, Crowther CA, et al. (Cochrane Pregnancy and Childbirth Group) (April 2016). "Regimens of fetal surveillance of suspected large-for-gestational-age fetuses for improving health outcomes". The Cochrane Database of Systematic Reviews. 4: CD011739. doi:10.1002/14651858.CD011739.pub2. PMC 7081118. PMID 27045604.
- ^ Miller V, Saxena S, Farhan M (2010). "Management of large-for-gestational-age pregnancy in non-diabetic women". The Obstetrician & Gynaecologist. 12 (4): 250–256. doi:10.1576/toag.12.4.250.27617.
