Butane (data page)
This page provides supplementary chemical data on n-butane.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source such as MSDS Search Engine, and follow its directions.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.3326 at 20°C[1] |
| Dielectric constant, εr | 1.7697 ε0 at 23°C[2] |
| Symmetry group | C2h |
| Magnetic susceptibility | ? |
| Surface tension | 12.46 dyn/cm at 20°C P ≈ 225 kPa |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Density (liquid) 0°C | 600 kg/m³ |
| Density (saturated vapor) 1 atm, -0.5°C | 2.6 kg/m³ |
| Triple point | 134.6 K (–138.5 °C), 0.7 Pa |
| Critical point | 425.1 K (152.0 °C), 3796.0 kPa |
| Std enthalpy change of fusion, ΔfusH |
4.66 kJ/mol |
| Std entropy change of fusion, ΔfusS |
34.56 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
22.44 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
82.30 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
-147.6 kJ/mol |
| Standard molar entropy, S |
229.7 J/(mol K) |
| Heat capacity, cp | 132.42 J/(mol K) –262°C to –3°C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
–125.6 kJ/mol |
| Standard molar entropy, S |
310.23 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–2877.5 kJ/mol |
| Heat capacity, cp | 98.49 J/(mol K) at 25°C |
| n-butane van der Waals' constants[3] |
a = 1466.2 L2 kPa/mol2 b = 0.1226 liter per mole |
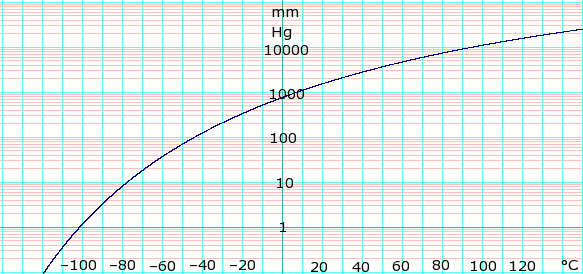
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 | |
| T in °C | –101.5 | –77.8 | –59.1 | –44.2 | –16.3 | –0.5 | 18.8 | 50.0 | 79.5 | 116.0 | — | — | |
n-Butane: Table data obtained from CRC Handbook of Chemistry and Physics 44th ed.
Spectral data
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
References
- "Multifunctional Green Alternative: Propane and Butane (properties)". Seminck. Retrieved 1 May 2007.
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.

