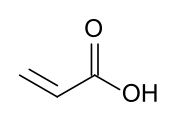
Acrylic acid
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Prop-2-enoic acid | |
| Other names
Acrylic acid
Acroleic acid Ethylenecarboxylic acid Propene acid Propenoic acid Vinylformic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.071 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H4O2 | |
| Molar mass | 72.063 g·mol−1 |
| Appearance | clear, colorless liquid |
| Odor | acrid[2] |
| Density | 1.051 g/mL |
| Melting point | 14 °C (57 °F; 287 K) |
| Boiling point | 141 °C (286 °F; 414 K) |
| Miscible | |
| Vapor pressure | 3 mmHg[2] |
| Acidity (pKa) | 4.25[3] |
| Viscosity | 1.3 cP at 20 °C (68 °F) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Corrosive (C), Dangerous for the environment (N) |
| NFPA 704 (fire diamond) | |
| Flash point | 68 °C (154 °F; 341 K) |
| 429 °C (804 °F; 702 K) | |
| Explosive limits | 2.4%-8.02%[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[2] |
REL (Recommended)
|
TWA 2 ppm (6 mg/m3) [skin][2] |
IDLH (Immediate danger)
|
N.D.[2] |
| Safety data sheet (SDS) | MSDS |
| Related compounds | |
Other anions
|
acrylate |
Related carboxylic acids
|
acetic acid propionic acid lactic acid 3-hydroxypropionic acid malonic acid butyric acid crotonic acid |
Related compounds
|
allyl alcohol propionaldehyde acrolein methyl acrylate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Acrylic acid (IUPAC: prop-2-enoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually.[4]
Production
Acrylic acid is produced from propylene which is a byproduct of ethylene and gasoline production.
- CH2=CHCH3 + 3⁄2 O2 → CH2=CHCO2H + H2O
Ethylene can be carboxylated to acrylic acid under supercritical carbon dioxide condition.[5]
Because acrylic acid and its esters have long been valued commercially, many other methods have been developed but most have been abandoned for economic or environmental reasons. An early method was the hydrocarboxylation of acetylene ("Reppe chemistry"):
- HCCH + CO + H2O → CH2=CHCO2H
This method requires nickel carbonyl and high pressures of carbon monoxide. It was once manufactured by the hydrolysis of acrylonitrile which is derived from propene by ammoxidation, but was abandoned because the method cogenerates ammonium derivatives. Other now abandoned precursors to acrylic acid include ethenone and ethylene cyanohydrin.[4]
Dow Chemical Company and a partner, OPX Biotechnologies, are investigating using fermented sugar to produce 3-hydroxypropionic acid (3HP), an acrylic acid precursor. [6] The goal is to reduce greenhouse gas emissions. [7]
Reactions and uses
Acrylic acid undergoes the typical reactions of a carboxylic acid. When reacted with an alcohol, it forms the corresponding ester. The esters and salts of acrylic acid are collectively known as acrylates (or propenoates). The most common alkyl esters of acrylic acid are methyl, butyl, ethyl, and 2-ethylhexyl acrylate.
Acrylic acid and its esters readily combine with themselves (to form polyacrylic acid) or other monomers (e.g. acrylamides, acrylonitrile, vinyl, styrene, and butadiene) by reacting at their double bond, forming homopolymers or copolymers which are used in the manufacture of various plastics, coatings, adhesives, elastomers, as well as floor polishes, and paints.
Substituents
As a substituent acrylic acid can be found as an acyl group or a carboxyalkyl group depending on the removal of the group from the molecule. More specifically these are:
- The acryloyl group, with the removal of the –OH from carbon-1.
- The 2-carboxyethenyl group, with the removal of a –H from carbon-3. This substituent group is found in chlorophyll.
Safety
Acrylic acid is severely irritating and corrosive to the skin and the respiratory tract. Eye contact can result in severe and irreversible injury. Low exposure will cause minimal or no health effects, while high exposure could result in pulmonary edema. The LD50 is 340 mg/kg (rat, oral).
See also
References
- ^ Merck Index, 11th Edition, 124.
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0013". National Institute for Occupational Safety and Health (NIOSH).
- ^ Dippy, J.F.J.; Hughes, S.R.C.; Rozanski, A. (1959). J. Chem Soc.: 2492.
{{cite journal}}: CS1 maint: untitled periodical (link) - ^ a b Takashi Ohara, Takahisa Sato, Noboru Shimizu, Günter Prescher Helmut Schwind, Otto Weiberg, Klaus Marten, Helmut Greim "Acrylic Acid and Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry 2003, Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_161.pub2
- ^ Sakakura, Toshiyasu; Choi, Jun-Chul; Yasuda, Hiroyuki (13 June 2007). "Transformation of Carbon dioxide". Chemical Reviews. 107 (6). American Chemical Society: 2365–2387. doi:10.1021/cr068357u. PMID 17564481.
- ^ Sweet Deal: Dow and Partner Cook up Sugar-to-Acrylic Plan. Durabilityanddesign.com. Retrieved on 2012-05-24.
- ^ Better Bugs to Make Plastics, Technology Review, September 20, 2010, retrieved January 9, 2012. Technologyreview.com (2010-09-20). Retrieved on 2012-05-24.

