Acylal
Appearance
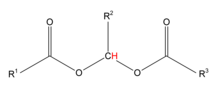
Acylals in organic chemistry are a group of chemical compounds sharing a functional group with the general structure RCH(OOCR)2. Acylals can be obtained by reaction of aldehydes with acetic anhydride and a suitable catalyst for instance with sulfated zirconia[1] at low temperatures when used as protective groups for aldehydes. High temperature exposure converts the acylal back to the aldehyde.
References
- ^ Negrón, Guillermo E.; Palacios, Laura N.; Angeles, Deyanira; Lomas, Leticia; Gaviño, Rubén (May–June 2005). "A mild and efficient method for the chemoselective synthesis of acylals from aromatic aldehydes and their deprotections catalyzed by sulfated zirconia". J. Braz. Chem. Soc. 16 (3a). São Paulo. doi:10.1590/S0103-50532005000300025.
