Α-Aminoadipate pathway
It has been suggested that Homoisocitrate be merged into this article. (Discuss) Proposed since April 2013. |
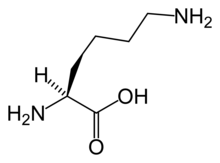
The α-aminoadipate pathway is a biochemical pathway for the synthesis of the amino acid L-lysine. In the eukaryotes, this pathway is unique to the higher fungi (containing chitin in their cell walls) and the euglenids.[1] It has also been reported from bacteria of the genus Thermus.[2]
Pathway overview
Homocitrate is initially synthesised from acetyl-CoA and 2-oxoglutarate by homocitrate synthase. This is then converted to homoaconitate by homoaconitase and then to homoisocitrate by homoisocitrate dehydrogenase. A nitrogen atom is added from glutamate by aminoadipate aminotransferase to form the α-aminoadipate from which this pathway gets its name. This is then reduced by aminoadipate reductase via an acyl-enzyme intermediate to a semialdehyde. Reaction with glutamate by one class of saccharopine dehydrogenase yields saccharopine which is then cleaved by a second saccharopine dehydrogenase to yield lysine and oxoglutarate.[3]
References
- ^ Zabriskie TM, Jackson MD. (2000). "Lysine biosynthesis and metabolism in fungi". Natural Product Reports. 17 (1): 85–97. doi:10.1039/a801345d. PMID 10714900.
- ^ Kosuge T, Hoshino T (1999). "The α-aminoadipate pathway for lysine biosynthesis is widely distributed among Thermus strains". Journal of Bioscience and Bioengineering. 88 (6): 672–5. doi:10.1016/S1389-1723(00)87099-1. PMID 16232683.
- ^ Xu H, Andi B, Qian J, West AH, Cook PF (2006). "The α-aminoadipate pathway for lysine biosynthesis in fungi". Cell Biochemistry and Biophysics. 46 (1): 43–64. doi:10.1385/CBB:46:1:43. PMID 16943623.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
