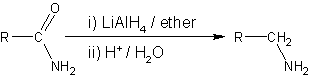Amide reduction
Amide reduction is a reaction in organic synthesis where an amide is reduced to either an amine or an aldehyde functional group.[1][2]
Catalytic hydrogenation
Catalytic hydrogenation can be used to reduce amides to amines; however, the process often requires high hydrogenation pressures and reaction temperatures to be effective (i.e. often requiring pressures above 197 atm and temperatures exceeding 200 °C).[1] Selective catalysts for the reaction include Copper chromite, Rhenium trioxide, and Rhenium(VII) oxide.
Non-catalytic routes to amines
Reducing agents able to affect this reaction include metal hydrides such as lithium aluminium hydride,[3][4][5][6][7] or lithium borohydride in mixed solvents of tetrahydrofuran and methanol,[8]
Non-catalytic routes to aldehydes
N,N-disubstituted amides can be reduced to aldehydes by using an excess of the amide:[citation needed]
- R(CO)NRR' + LiAlH4 → RCHO + HNRR'
With further reduction the alcohol is obtained.
Some amides can be reduced to aldehydes in the Sonn-Müller method.
Hydrosilylation
A well known method for amide reduction is hydrosilylation with silyl hydrides and a suitable catalyst based on Rh, Ru, Pt, Pd, Ir, Os, Re, Mn, Mo, In, or Ti.[citation needed]
Iron catalysis by triiron dodecacarbonyl in combination with polymethylhydrosiloxane has been reported.[9]
References
- ^ a b Nishimura, Shigeo (2001). Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (1st ed.). New York: Wiley-Interscience. pp. 406–411. ISBN 9780471396987.
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595
- ^ Cope, Arthur C.; Ciganek, Engelbert (1959). "N,N-Dimethylcyclohexylmethylamine". Organic Syntheses. 39: 19. doi:10.15227/orgsyn.039.0019.
- ^ Wilson, C. V.; Stenberg, J. F. (1956). "Laurylmethylamine". Organic Syntheses. 36: 48. doi:10.15227/orgsyn.036.0048.
- ^ Moffett, Robert Bruce (1953). "2,2-Dimethylpyrrolidine". Organic Syntheses. 33: 32. doi:10.15227/orgsyn.033.0032.
- ^ Park, Chung Ho; Simmons, Howard E. (1974). "Macrocyclic Diimines: 1,10-Diazacylooctadecane". Organic Syntheses. 54: 88. doi:10.15227/orgsyn.054.0088.
- ^ Seebach, Dieter; Kalinowski, Hans-Otto; Langer, Werner; Crass, Gerhard; Wilka, Eva-Maria (1983). "Chiral Media for Asymmetric Solvent Inductions". Organic Syntheses. 61: 24. doi:10.15227/orgsyn.061.0024.
- ^ Ookawa, Atsuhiro; Soai, Kenso (1986). "Mixed solvents containing methanol as useful reaction media for unique chemoselective reductions within lithium borohydride". The Journal of Organic Chemistry. 51 (21): 4000–4005. doi:10.1021/jo00371a017.
- ^ Zhou, S.; Junge, K.; Addis, D.; Das, S.; Beller, M. (2009). "A Convenient and General Iron-Catalyzed Reduction of Amides to Amines". Angewandte Chemie International Edition in English. 48 (50): 9507–9510. doi:10.1002/anie.200904677. PMID 19784999.