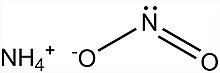
Ammonium nitrite
| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.257 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NH4NO2 | |
| Molar mass | 64.06 g/mol |
| Appearance | pale yellow crystals, slowly decomposes to nitrogen and water |
| Density | 1.69 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ammonium nitrite, NH4NO2, is the ammonia salt of nitrous acid. It is not used in pure isolated form, since it is highly unstable and decomposes even at ordinary temperature.
Preparation
Ammonium nitrite forms naturally in the air and can be prepared by the absorption of equal parts nitrogen dioxide and nitric oxide upon aqueous ammonia.[1]
It can also be prepared by oxidizing ammonia with ozone or hydrogen peroxide, or in a precipitation reaction of barium or lead nitrite with ammonium sulfate, or silver nitrite with ammonium chloride, or ammonium perchlorate with potassium nitrite. The precipitate is filtered off and the solution concentrated. It forms colorless crystals which are soluble in water and decompose on heating or in the presence of acid, with the formation of nitrogen.[2] Ammonium nitrite solution is stable at higher pH and lower temperature. If there is any decrease in pH lower than 7.0, It may lead to explosion. It is desirable to maintain pH by adding ammonia solution. The mole ratio of Ammonium Nitrite to Ammonia must be above 10% mole ratio.
- NH4NO2 → N2 + 2 H2O
Properties
Ammonium nitrite may explode at a temperature of 60–70 °C,[1] and will decompose quicker when dissolved in a concentrated aqueous solution, than in the form of a dry crystal.
References
- ^ a b Thomas Scott; Mary Eagleson (1994). Concise encyclopedia chemistry. Walter de Gruyter. p. 66. ISBN 3-11-011451-8.
- ^ "VIAS Encyclopedia: Ammonium Nitrite".
