Ammonium thiosulfate
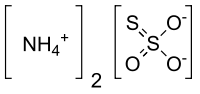
| |
| Names | |
|---|---|
| IUPAC name
Diammonium thiosulfate
| |
| Other names
Ammonium thiosulphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.074 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H8N2O3S2 | |
| Molar mass | 148.20 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ammonium thiosulfate (ammonium thiosulphate in British English) is an inorganic compound. It is white crystalline solid with ammonia odor, readily soluble in water, slightly soluble in acetone and insoluble in ethanol and diethyl ether.[1]
Applications
Photographic fixation
Ammonium thiosulfate is used in photographic fixer. It is a so-called rapid fixer, acting more quickly than sodium thiosulfate fixers.[2] Fixation involves these chemical reactions (illustrated for silver bromide):[3]
- AgBr + 2 (NH4)2S2O3 → (NH4)3[Ag(S2O3)2] + NH4Br
- AgBr + 3 (NH4)2S2O3 → (NH4)5[Ag(S2O3)3] + NH4Br
Extractive metallurgy
Ammonium thiosulfate is also used for leaching of gold and silver. It works with presence of copper as a catalyst here. This process is a nontoxic alternative gold cyanidation.[4]
Other
Ammonium thiosulfate can be used as a fertilizer.[5] As suggested by some research studies it can be used as an additive to coal-waste mixtures to reduce formation of very dangerous dioxins and furans.[6]
See also
References
- ^ MSDS - Ammonium Thiosulfate
- ^ Praní černobílých filmů a papírů
- ^ Karlheinz Keller et al. "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a20_001
- ^ Thiosulfate leaching of gold — A review
- ^ Minerální hnojiva se sírou
- ^ Ekologický monitor. Krátké zprávy ze zahraničních periodik – 19.1.2006
