Aspergillus
| Aspergillus | |
|---|---|

| |
| Conidial head of Aspergillus niger | |
| Scientific classification | |
| Kingdom: | |
| Division: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | Aspergillus Micheli (1729)
|
| Species | |
Aspergillus (/ˌæspərˈdʒɪləs/) is a genus consisting of a few hundred mould species found in various climates worldwide.
Aspergillus was first catalogued in 1729 by the Italian priest and biologist Pier Antonio Micheli. Viewing the fungi under a microscope, Micheli was reminded of the shape of an aspergillum (holy water sprinkler), from Latin spargere (to sprinkle), and named the genus accordingly.[1] Today, aspergillum is also the name of an asexual spore-forming structure common to all Aspergillus species; around one-third of species are also known to have a sexual stage.[2]
Taxonomy
Species
Aspergillus consists of a few hundred species.[2]
Growth and distribution

Aspergillus is defined as a group of conidial fungi—that is, fungi in an asexual state. Some of them, however, are known to have a teleomorph (sexual state) in the Ascomycota, so with DNA evidence forthcoming, members of the genus Aspergillus can tentatively be considered members of the Ascomycota.
Members of the genus possess the ability to grow where a high osmotic concentration (high sugar, salt, etc.) exists. Aspergillus species are highly aerobic and are found in almost all oxygen-rich environments, where they commonly grow as molds on the surface of a substrate, as a result of the high oxygen tension. Commonly, fungi grow on carbon-rich substrates like monosaccharides (such as glucose) and polysaccharides (such as amylose). Aspergillus species are common contaminants of starchy foods (such as bread and potatoes), and grow in or on many plants and trees.[citation needed]
In addition to growth on carbon sources, many species of Aspergillus demonstrate oligotrophy where they are capable of growing in nutrient-depleted environments, or environments with a complete lack of key nutrients. A. niger is a prime example of this; it can be found growing on damp walls, as a major component of mildew.
Commercial importance


Species of Aspergillus are important medically and commercially. Some species can cause infection in humans and other animals. Some infections found in animals have been studied for years, while other species found in animals have been described as new and specific to the investigated disease, and others have been known as names already in use for organisms such as saprophytes. More than 60 Aspergillus species are medically relevant pathogens.[3] For humans, a range of diseases such as infection to the external ear, skin lesions, and ulcers classed as mycetomas are found.
Other species are important in commercial microbial fermentations. For example, alcoholic beverages such as Japanese sake are often made from rice or other starchy ingredients (like manioc), rather than from grapes or malted barley. Typical microorganisms used to make alcohol, such as yeasts of the genus Saccharomyces, cannot ferment these starches. Therefore, koji mold such as Aspergillus oryzae is used to first break down the starches into simpler sugars.[citation needed]
Members of the genus are also sources of natural products that can be used in the development of medications to treat human disease.[4]
Perhaps the largest application of A. niger is as the major source of citric acid; this organism accounts for over 99% of global citric acid production, or more than 1.4 million tonnes per year.[citation needed] A. niger is also commonly used for the production of native and foreign enzymes, including glucose oxidase, lysozyme, and lactase (see section 2 in http://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/UCM400718). In these instances, the culture is rarely grown on a solid substrate, although this is still common practice in Japan, but is more often grown as a submerged culture in a bioreactor. In this way, the most important parameters can be strictly controlled, and maximal productivity can be achieved. This process also makes it far easier to separate the chemical or enzyme of importance from the medium, and is therefore far more cost-effective.
Research


A. nidulans (Emericella nidulans) has been used as a research organism for many years and was used by Guido Pontecorvo to demonstrate parasexuality in fungi. Recently, A. nidulans was one of the pioneering organisms to have its genome sequenced by researchers at the Broad Institute. As of 2008, a further seven Aspergillus species have had their genomes sequenced: the industrially useful A. niger (two strains), A. oryzae, and A. terreus, and the pathogens A. clavatus, A. fischerianus (Neosartorya fischeri), A. flavus, and A. fumigatus (two strains).[5] A. fischerianus is hardly ever pathogenic, but is very closely related to the common pathogen A. fumigatus; it was sequenced in part to better understand A. fumigatus pathogenicity.[6]
Sexual reproduction
Of the 250 species of aspergilli, about 64% have no known sexual state.[7] However, many of these species likely have an as yet unidentified sexual stage.[7] Sexual reproduction occurs in two fundamentally different ways in fungi. These are outcrossing (in heterothallic fungi) in which two different individuals contribute nuclei, and self-fertilization or selfing (in homothallic fungi) in which both nuclei are derived from the same individual. In recent years, sexual cycles have been discovered in numerous species previously thought to be asexual. These discoveries reflect recent experimental focus on species of particular relevance to humans.
A. fumigatus is the most common species to cause disease in immunodeficient humans. In 2009, A. fumigatus was shown to have a heterothallic, fully functional sexual cycle.[8] Isolates of complementary mating types are required for sex to occur.
A. flavus is the major producer of carcinogenic aflatoxins in crops worldwide. It is also an opportunistic human and animal pathogen, causing aspergillosis in immunocompromised individuals. In 2009, a sexual state of this heterothallic fungus was found to arise when strains of opposite mating types were cultured together under appropriate conditions.[9]
A. lentulus is an opportunistic human pathogen that causes invasive aspergillosis with high mortality rates. In 2013, A. lentulus was found to have a heterothallic functional sexual breeding system.[10]
A. terreus is commonly used in industry to produce important organic acids and enzymes, and was the initial source for the cholesterol-lowering drug lovastatin. In 2013, A. terreus was found to be capable of sexual reproduction when strains of opposite mating types were crossed under appropriate culture conditions.[11]
These findings with Aspergillus species are consistent with accumulating evidence, from studies of other eukaryotic species, that sex was likely present in the common ancestor of all eukaryotes.[12][13][14]
A. nidulans, a homothallic fungus, is capable of self-fertilization. Selfing involves activation of the same mating pathways characteristic of sex in outcrossing species, i.e. self-fertilization does not bypass required pathways for outcrossing sex, but instead requires activation of these pathways within a single individual.[15]
Among those Aspergillus species that exhibit a sexual cycle, the overwhelming majority in nature are homothallic (self-fertilizing).[16] This observation suggests Aspergillus species can generally maintain sex though little genetic variability is produced by homothallic self-fertilization. A. fumigatus, a heterothallic (outcrossing) fungus that occurs in areas with widely different climates and environments, also displays little genetic variability either within geographic regions or on a global scale,[17] again suggesting sex, in this case outcrossing sex, can be maintained even when little genetic variability is produced.
Genomics
The simultaneous publication of three Aspergillus genome manuscripts in Nature in December 2005 established the genus as the leading filamentous fungal genus for comparative genomic studies. Like most major genome projects, these efforts were collaborations between a large sequencing centre and the respective community of scientists. For example, the Institute for Genome Research (TIGR) worked with the A. fumigatus community. A. nidulans was sequenced at the Broad Institute. A. oryzae was sequenced in Japan at the National Institute of Advanced Industrial Science and Technology. The Joint Genome Institute of the Department of Energy has released sequence data for a citric acid-producing strain of A. niger. TIGR, now renamed the Venter Institute, is currently spearheading a project on the A. flavus genome.[18]
Genome sizes for sequenced species of Aspergillus range from about 29.3 Mb for A. fumigatus to 37.1 Mb for A. oryzae, while the numbers of predicted genes vary from about 9926 for A. fumigatus to about 12,071 for A. oryzae. The genome size of an enzyme-producing strain of A. niger is of intermediate size at 33.9 Mb.[1]
Pathogens
Some Aspergillus species cause serious disease in humans and animals. The most common pathogenic species are A. fumigatus and A. flavus, which produces aflatoxin which is both a toxin and a carcinogen, and which can contaminate foods such as nuts. The most common species causing allergic disease are A. fumigatus and A. clavatus. Other species are important as agricultural pathogens. Aspergillus spp. cause disease on many grain crops, especially maize, and some variants synthesize mycotoxins, including aflatoxin. Aspergillus can cause neonatal infections.[19]
A. fumigatus (the most common species) infections are primary pulmonary infections and can potentially become a rapidly necrotizing pneumonia with a potential to disseminate. The organism can be differentiated from other common mold infections based on the fact that it takes on a mold form both in the environment and in the host (unlike Candida albicans which is a dimorphic mold in the environment and a yeast in the body).
Aspergillosis
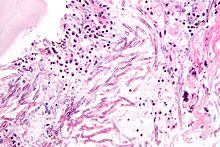
Aspergillosis is the group of diseases caused by Aspergillus. The most common subtype among paranasal sinus infections associated with aspergillosis is A. fumigatus.[20] The symptoms include fever, cough, chest pain, or breathlessness, which also occur in many other illnesses, so diagnosis can be difficult. Usually, only patients with already weakened immune systems or who suffer other lung conditions are susceptible.
In humans, the major forms of disease are:[21][22]
- Allergic bronchopulmonary aspergillosis, which affects patients with respiratory diseases such as asthma, cystic fibrosis, and sinusitis
- Acute invasive aspergillosis, a form that grows into surrounding tissue, more common in those with weakened immune systems such as AIDS or chemotherapy patients
- Disseminated invasive aspergillosis, an infection spread widely through the body
- Aspergilloma, a "fungus ball" that can form within cavities such as the lung
Aspergillosis of the air passages is also frequently reported in birds, and certain species of Aspergillus have been known to infect insects.[3]
See also
- List of Aspergillus species
- Mold health issues
- New England Compounding Center meningitis outbreak
- Sick building syndrome
References
- ^ a b Bennett JW (2010). "An Overview of the Genus Aspergillus" (PDF). Aspergillus: Molecular Biology and Genomics. Caister Academic Press. ISBN 978-1-904455-53-0.
- ^ a b Geiser, D. (2009). "Sexual structures in Aspergillus: morphology, importance and genomics". Medical mycology : official publication of the International Society for Human and Animal Mycology. 47. Suppl 1 (s1): S21–S26. doi:10.1080/13693780802139859. PMID 18608901.
- ^ a b Thom, C; Church, M (1926). The Aspergilli. Baltimore: The Williams & Wilkins Company.
- ^ US 6069146
- ^ Wortman; Gilsenan, J.; Joardar, V.; Deegan, J.; Clutterbuck, J.; Andersen, M.; Archer, D.; Bencina, M.; Braus, G.; Coutinho, P.; Von Döhren; Doonan, J.; Driessen, A. J.; Durek, P.; Espeso, E.; Fekete, E.; Flipphi, M.; Estrada, C. G.; Geysens, S.; Goldman, G.; De Groot; Hansen, K.; Harris, S. D.; Heinekamp, T.; Helmstaedt, K.; Henrissat, B.; Hofmann, G.; Homan, T.; Horio, T.; Horiuchi, H. (2009). "The 2008 update of the Aspergillus nidulans genome annotation: a community effort". Fungal Genetics and Biology. 46. Suppl 1 (1): S2–S13. doi:10.1016/j.fgb.2008.12.003. PMC 2826280. PMID 19146970.
- ^ "Descriptions – Aspergillus Comparative". Broad Institute. Archived from the original on 22 November 2009. Retrieved 2009-10-15.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Dyer PS, O'Gorman CM (December 2011). "A fungal sexual revolution: Aspergillus and Penicillium show the way". Curr. Opin. Microbiol. 14 (6): 649–54. doi:10.1016/j.mib.2011.10.001. PMID 22032932.
- ^ O'Gorman CM, Fuller H, Dyer PS (January 2009). "Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus". Nature. 457 (7228): 471–4. doi:10.1038/nature07528. PMID 19043401.
- ^ Horn BW, Moore GG, Carbone I (2009). "Sexual reproduction in Aspergillus flavus". Mycologia. 101 (3): 423–9. doi:10.3852/09-011. PMID 19537215.
- ^ Swilaiman SS, O'Gorman CM, Balajee SA, Dyer PS (July 2013). "Discovery of a sexual cycle in Aspergillus lentulus, a close relative of A. fumigatus". Eukaryotic Cell. 12 (7): 962–9. doi:10.1128/EC.00040-13. PMC 3697472. PMID 23650087.
- ^ Arabatzis M, Velegraki A (2013). "Sexual reproduction in the opportunistic human pathogen Aspergillus terreus". Mycologia. 105 (1): 71–9. doi:10.3852/11-426. PMID 23074177.
- ^ Malik SB, Pightling AW, Stefaniak LM, Schurko AM, Logsdon JM (2008). "An expanded inventory of conserved meiotic genes provides evidence for sex in Trichomonas vaginalis". PLoS ONE. 3 (8): e2879. doi:10.1371/journal.pone.0002879. PMC 2488364. PMID 18663385.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Bernstein H and Bernstein C (2013). Evolutionary Origin and Adaptive Function of Meiosis. In Meiosis: Bernstein C and Bernstein H, editors. ISBN 978-953-51-1197-9, InTech, http://www.intechopen.com/books/meiosis/evolutionary-origin-and-adaptive-function-of-meiosis
- ^ Heitman J, Sun S, James TY (2013). "Evolution of fungal sexual reproduction". Mycologia. 105 (1): 1–27. doi:10.3852/12-253. PMID 23099518.
- ^ Paoletti M, Seymour FA, Alcocer MJ, Kaur N, Calvo AM, Archer DB, Dyer PS (August 2007). "Mating type and the genetic basis of self-fertility in the model fungus Aspergillus nidulans". Curr. Biol. 17 (16): 1384–9. doi:10.1016/j.cub.2007.07.012. PMID 17669651.
- ^ Dyer PS, O'Gorman CM (January 2012). "Sexual development and cryptic sexuality in fungi: insights from Aspergillus species". FEMS Microbiol. Rev. 36 (1): 165–92. doi:10.1111/j.1574-6976.2011.00308.x. PMID 22091779.
- ^ Rydholm C, Szakacs G, Lutzoni F (April 2006). "Low genetic variation and no detectable population structure in aspergillus fumigatus compared to closely related Neosartorya species". Eukaryotic Cell. 5 (4): 650–7. doi:10.1128/EC.5.4.650-657.2006. PMC 1459663. PMID 16607012.
- ^ Machida, M; Gomi, K, eds. (2010). Aspergillus: Molecular Biology and Genomics. Caister Academic Press. ISBN 978-1-904455-53-0.
- ^ Cloherty, John (2012). Manual of neonatal care. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. ISBN 978-1-60831-777-6; Access provided by the University of Pittsburgh.
{{cite book}}: CS1 maint: postscript (link) - ^ Bozkurt, MK; Ozçelik, T; Saydam, L; Kutluay, L (2008). "[A case of isolated aspergillosis of the maxillary sinus]". Kulak burun bogaz ihtisas dergisi (in Turkish). 18 (1): 53–5. PMID 18443405.
- ^ "Aspergillosis". MedScape.
- ^ Wilson WR et al. Current diagnosis and treatment in Infect Dis. Lange, 2001
- Du C, Lin SK, Koutinas A, Wang R, Dorado P, Webb C (Nov 2008). "A wheat biorefining strategy based on solid-state fermentation for fermentative production of succinic acid". Bioresour Technol. 99 (17): 8310–5. doi:10.1016/j.biortech.2008.03.019. PMID 18434138Template:Inconsistent citations
{{cite journal}}: CS1 maint: postscript (link) - Zirbes JM, Milla CE (Jun 2008). "Steroid-sparing effect of omalizumab for allergic bronchopulmonary aspergillosis and cystic fibrosis". Pediatr Pulmonol. 43 (6): 607–10. doi:10.1002/ppul.20804. PMID 18433040Template:Inconsistent citations
{{cite journal}}: CS1 maint: postscript (link) - Asan A. (February 10, 2015) [2004]. "Aspergillus, Penicillium, and Related Species Reported from Turkey" (PDF). Mycotaxon. 89 (1): 155–7Template:Inconsistent citations
{{cite journal}}: CS1 maint: postscript (link)
External links
- FungiDB: An integrated functional genomics database for fungi and oomycetes
- Aspergillus Genome Resources (NIH)
- Aspergillus Comparative Database Comparative genomic resource at the Broad Institute
- Central Aspergillus Data Repository
- The Fungal Genetics Stock Center
- The Aspergillus/Aspergillosis Website An encyclopedia of Aspergillus for patients, doctors and scientists
- Aspergillus surveillance project at a large tertiary-care hospital. (PDF).
- The Aspergillus Genome Database
