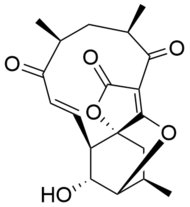
Atrop-abyssomicin C
| |
| Names | |
|---|---|
| IUPAC name
12,14a,3-(Epoxymethyno)-2H-1-benzoxacyclododecin-2,4,8(5H,10aH)-trione, 6,7,11,12,13,14-hexahydro-11-hydroxy-5,7,13-trimethyl-, (5R,7S,9E,10aR,11R,12R,13R,14aR)
| |
| Other names
Atrop-abyssomicin C
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C19H22O6 | |
| Molar mass | 346.38 g/mol |
| Density | 1.34±0.1 g/cm3 (Predicted) |
| Melting point | 180 °C (decomp) |
| Boiling point | 597.5±50.0 °C (Predicted) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Atrop-abyssomicin C is a polycyclic polyketide-type natural product that is the atropisomer of abyssomicin C. It is a spirotetronate that belongs to the class of tetronate antibiotics, which includes compounds such as tetronomycin, agglomerin, and chlorothricin.[1] In 2006, the Nicolaou group discovered atrop-abyssomicin C while working on the total synthesis of abyssomicin C.[2] Then in 2007, Süssmuth and co-workers isolated atrop-abyssomicin C from Verrucosispora maris AB-18-032, a marine actinomycete found in sediment of the Japanese sea. They found that atrop-abyssomicin C was the major metabolite produced by this strain, while abyssomicin C was a minor product. The molecule displays antibacterial activity by inhibiting the enzyme PabB (4-amino-4-deoxychorismate synthase), thereby depleting the biosynthesis of p-aminobenzoate.[3][4]
Structure[edit]
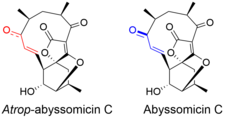
Atrop-abyssomicin C has a complex, yet intriguing structural topography. The compound contains an oxabicyclo[2.2.2]octane system fused to the tetronate moiety. The 11-membered macrocyclic ring carries an α,β-unsaturated ketone that was proposed to be the reactive center.[5] Despite being a strained macrocycle, there exist an atropisomer, abyssomicin C. The atropisomerism arise due to a structural deviation in the α,β-unsaturated ketone region of the molecule. The orientation of the carbonyl in atrop-abyssomicin C is cisoid, whereas the conformation in abyssomicin C is transoid.[6] The enone moiety of atrop-abyssomicin C has a higher degree of the conjugation, which makes it a more active Michael acceptor.[7]
Biosynthesis[edit]
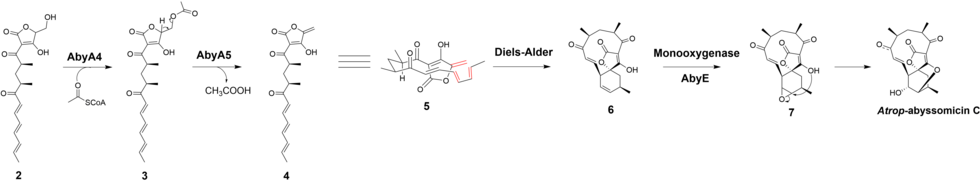
The biosynthesis of atrop-abyssomicin C begins with the synthesis of a linear polyketide chain in a PKS I system that consist of one loading and six extension modules. The polyketide chain is made from five acetates, two propionates, and the glycolytic pathway metabolite. D-1,3-bisphosphoglycerate, the glycolytic metabolite, is transferred to AbyA3 (an acyl-carrier protein) by AbyA2 to generate the glyceryl-ACP. AbyA1 facilitates the attachment of the glyceryl-ACP to the polyketide chain and the detachment of the polyketide from the polyketide synthase to form intermediate 2.[7][8][9]

Based on the observation made for the biosynthesis of agglomerin, it has been proposed that AbyA4 acetylates intermediate 2 and AbyA5 catalyzes the elimination of acetic acid to form the exocyclic double bond in intermediate 4.[1] An intramolecular Diels-Alder was proposed to take place between the exocyclic olefin and the conjugated diene at the tail end of the polyketide to form the macrocyclic ring.[7] It has been reported that the previously unidentified Abycyc gene could code for an enzyme that carries out the Diels-Alder cycloaddition.[10] Following the Diels-Alder reaction, an epoxide ring is formed and then opened by the tetronate hydroxyl group to form atrop-abyssomicin C. It has been postulated that the AbyE monooxygenase catalyzes epoxide formation.[8]
References[edit]
- ^ a b Kanchanabanca C, Tao, W., Hong, H., Liu, Y., Hanh, F., Samborskyy, M., Deng, Z., Sun, Y., Leadlay, P.F. (2013). "Unusual acetylation-elimination in the formation of tetronate antibiotics". Angewandte Chemie International Edition. 52 (22): 5785–8. doi:10.1002/anie.201301680. PMID 23606658.
- ^ Nicolaou K, Harrison, S.T. (2006). "Total Synthesis of Abyssomicin C and atrop-Abyssomicin C**". Angewandte Chemie International Edition. 45 (20): 3256–60. doi:10.1002/anie.200601116. PMID 16634106.
- ^ Keller S, Nicholson, G., Drahl, C., Sorensen, E., Fiedler, H.P., Süssmuth, R.D. (2007). "Abyssomicins G and H and atrop-abyssomicin C from the marine Verrucosispora strain AB-18-032". Journal of Antibiotics. 60 (6): 391–4. doi:10.1038/ja.2007.54. PMID 17617698.
- ^ Keller S, Schadt HS, Ortel I, Süssmuth RD (2007). "Action of atrop-abyssomicin C as an inhibitor of 4-amino-4-deoxychorismate synthase PabB". Angew. Chem. Int. Ed. Engl. 46 (43): 8284–6. doi:10.1002/anie.200701836. PMID 17886307.
- ^ Nicolaou K, Harrison, S.T., Chen, J.S. (2009). "Discoveries from the Abyss: The Abyssomicins and Their Total Synthesis". Synthesis. 2009 (1): 33–42. doi:10.1055/s-0028-1083259. PMC 2677807. PMID 20047014.
- ^ Nicolaou K, Harrison, S.T. (2007). "Total synthesis of abyssomicin C, atrop-abyssomicin C, and abyssomicin D: implications for natural origins of atrop-abyssomicin C". Journal of the American Chemical Society. 129 (2): 429–40. doi:10.1021/ja067083p. PMID 17212423.
- ^ a b c Savic V (2013). "Chapter 5 – Abyssomicins: Isolation, Properties, and Synthesis". Studies in Natural Products Chemistry. 40: 133–172. doi:10.1016/B978-0-444-59603-1.00005-9.
- ^ a b Gottardi E, Krawczyk, J., von Suchodoletz, H., Schadt, S., Mühlenweg, A., Uguru, G., Süssmuth, R.D. (2011). "Abyssomicin Biosynthesis: Formation of an Unusual Polyketide, Antibiotic-Feeding Studies and Genetic Analysis". ChemBioChem. 12 (9): 1401–1410. doi:10.1002/cbic.201100172. PMC 3625739. PMID 21656887.
- ^ Vieweg L, Reichau, S., Schobert, R, Leadlay, P.F., Süssmuth, R. (2014). "Recent advances in the field of bioactive tetronates". Natural Product Reports. 31 (11): 1554–1584. doi:10.1039/c4np00015c. PMID 24965099.
- ^ Hashimoto T, Hashimoto, J., Teruya, K., Hirano, T., Shin-ya, K., Ikeda, H., Liu, H.W., Nishiyama, M., Kuzuyama T. (2015). "Biosynthesis of versipelostatin: identification of an enzyme-catalyzed [4+2]-cycloaddition required for macrocyclization of spirotetronate-containing polyketides". Journal of the American Chemical Society. 137 (2): 572–5. doi:10.1021/ja510711x. PMC 4308742. PMID 25551461.
