Atropisomer

Atropisomers are stereoisomers arising because of hindered rotation about a single bond, where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual conformers.[1][2] The word atropisomer (Gr., άτροπος, atropos, meaning "without turn") was coined in application to a theoretical concept by German biochemist Richard Kuhn for Karl Freudenberg's seminal Stereochemie volume in 1933.[3] Atropisomerism was first experimentally detected in a tetra substituted biphenyl, a diacid, by George Christie and James Kenner in 1922.[4] Michinori Ōki further refined the definition of atropisomers taking into account the temperature-dependence associated with the interconversion of conformers, specifying that atropisomers interconvert with a half-life of at least 1000 seconds at a given temperature, corresponding to an energy barrier of 93 kJ mol−1 (22 kcal mol −1) at 300 K (27 °C).[5][6]
Three basic factors contribute to the stability of individual atropisomers: the repulsive interactions (e.g., steric bulk) of substituents near the axis of rotation, the length and rigidity of the single bond, a largely sp2-sp2 type of bond joining the aryl rings, and whether there are photochemical or other mechanisms to induce rotation in addition to thermal pathways.[1][6] A variety of methods are employed to study atropisomers, including (from more general to more specific/structural), dipolemetry, titrimetry, electronic and infrared spectroscopy, and X-ray crystallography and nuclear magnetic resonance spectroscopy, the last two being primary means of structure characterization of organic systems, and the last being an ideal means of studying dynamics when the system is amenable to it;[6] inferences from theory and results of reaction outcomes and yields also contribute.[7]
The importance of atropisomers arises to significant degree because with sufficient stability of a conformer, they can display axial chirality (planar chirality). Atropisomers that display axial chirality often have substituents ortho to the bond joining the aryl rings, substituents that cause significant steric repulsion that hinders rotation about the bond. The degree of hindrance correlates with the van der Waals radii of the particular substituents, and other properties that contribute to their repulsive potentials.[1] As the Ōki refinement of the atropisomer definition suggests, atropisomers are involved in a chemical equilibrium that, for a given structure, is thermally controlled; they differ in this way from most other types of chiral structures, where interconversion involves a chemical isomerization (i.e., with breaking and reforming of covalent bonds).
Stereochemical assignment
Determining the axial stereochemistry of biaryl atropisomers can be accomplished through the use of a Newman projection along the axis of hindered rotation. The ortho, and in some cases meta substituents are first assigned priority based on Cahn–Ingold–Prelog priority rules. Starting with the substituent of highest priority in the closest ring and moving along the shortest path to the substituent of highest priority in the other ring, the absolute configuration is assigned P for clockwise and M for counterclockwise. In the example shown, A has priority over B.[1]


Synthesis
One way to synthesize these axially chiral biaryl compounds is through a direct atroposelective coupling e.g. Ullmann coupling, Suzuki-Miyaura reaction, or palladium-catalyzed arylation of arenes.[8] Two methods of achieving diastereoselective coupling are through the use of a chiral bridge that links the two aryl groups or through the use of a chiral auxiliary at one of the positions proximal to axial bridge. Enantioselective coupling can be achieved through the use of a chiral leaving group on one of the biaryls or under oxidative conditions that utilize chiral amines to set the axial configuration.[1] Another method of synthesizing atropisomers is through the use of aromatic amides and thermodynamic control. By utilizing the planar rigid amide bond as seen in amino acids, and adding larger groups to the ortho position, chemists have been able to synthesize single atropisomers. Since atropisomers are thermally dependent, the thermodynamic control allows for selective synthesis under optimal conditions [9]
Scope
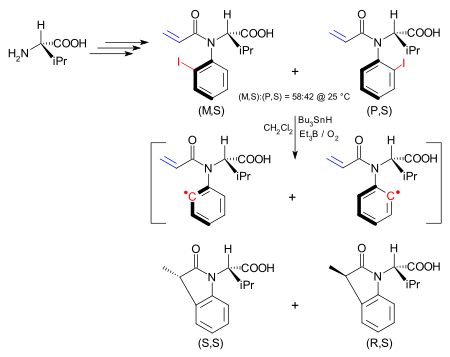


In one application the asymmetry in an atropisomer is transferred in a chemical reaction to a new stereocenter.[10] The atropisomer is an iodoaryl compound synthesised starting from (S)-valine and exists as the (M,S) isomer and the (P,S) isomer. The interconversion barrier between the two is 24.3 kcal/mol (101.7 kJ/mol). The (M,S) isomer can be obtained exclusively from this mixture by recrystallisation from hexanes. The iodine group is homolytically removed to form an aryl radical by a tributyltin hydride/triethylboron/oxygen mixture as in the Barton-McCombie reaction. Although the hindered rotation is now removed in the aryl radical, the intramolecular reaction with the alkene is so much faster than is rotation of the carbon-nitrogen bond that the stereochemistry is preserved. In this way the (M,S) isomer yields the (S,S) dihydroindolone.
The most important class of atropisomers are biaryls such as diphenic acid, which is a derivative of biphenyl with a complete set of ortho substituents. Heteroaromatic analogues of the biphenyl compounds also exist, where hindered rotation occurs about a carbon-nitrogen or a nitrogen-nitrogen bond.[6] Others are dimers of naphthalene derivatives such as 1,1'-bi-2-naphthol. In a similar way, aliphatic ring systems like cyclohexanes linked through a single bond may display atropisomerism provided that bulky substituents are present. The use of axially chiral biaryl compounds such as BINAP, QUINAP and BINOL, have been found to be useful in the area of asymmetric catalysis as chiral ligands.
Their ability to provide stereoinduction has led to use in metal catalyzed hydrogenation, epoxidation, addition, and allylic alkylation reactions.[1] Other reactions that can be catalyzed by the use of chiral biaryl compounds are the Grignard reaction, Ullmann reaction, and the Suzuki reaction.[11] A recent example in the area of chiral biaryl asymmetric catalysis employs a five-membered imidazole as part of the atropisomer scaffold. This specific phosphorus, nitrogen-ligand has been shown to perform enantioselective A3-coupling.[12]
Natural products, drug design
-
Mastigophorene A
-
(−)-N-Acetylallocolchinol
Many atropisomers occur in nature. Some natural products can be used as drugs and an example of this is mastigophorene A. Mastigophorene A has been found to aid in nerve growth.[1][13] Other examples of naturally occurring atropisomers include vancomycin isolated from an Actinobacterium, and knipholone, which is found in the roots of Kniphofia foliosa of the family Asphodelaceae. The structure complexity in vancomycin is significant because it can bind with peptides due to the complexity of its stereochemistry, which includes multiple stereocenters, two chiral planes in its stereogenic biaryl axis. Knipholone, with its axial chirality, occurs in nature and has been shown to offer good antimalarial and antitumor activities particularly in the M form.[1]
The pharmaceutical industry focuses its energy on producing enantiomerically pure compounds to be used as drugs. The use of atropisomers in synthesizing drugs allows for more stereochemical control.[14] One example is (−)-N-acetylallocolchinol, a drug that was discovered to aid in chemotherapy cancer treatment.[14][15]
Telenzepine is atropisomeric, in other words the molecule has a stereogenic C–N-axis in neutral aqueous solution it displays a half-life for racemization of the order of 1000 years. The enantiomers have been resolved. The activity is related to the (+)-isomer which is about 500-fold more active than the (–)-isomer at muscarinic receptors in rat cerebal cortex.[16] However, drug design is not always aided by atropisomerism. In some cases, making drugs from atropisomers is challenging because isomers may interconvert faster than expected. Atropisomers also might interact differently in the body, and as with other types of stereoisomers, it is important to examine these properties before administering drugs to patients.[16]
Further reading
- Allen K (November 14, 2005). "Atropisomerism: Axial Chirality in Nature and Synthesis" (PDF). Literature Seminar, Stoltz Research Group. California Institute of Technology.
References
- ^ a b c d e f g h Bringmann G, Mortimer AJP, Keller PA, Gresser MJ, Garner J, Breuning M (2005). "Atroposelective Synthesis of Axially Chiral Biaryl Compounds". Angewandte Chemie International Edition. 44 (34): 5384–5427. doi:10.1002/anie.200462661. PMID 16116589.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Anslyn and Dougherty, Modern Physical Organic Chemistry, University Science Books, 2006, ISBN 978-1-891389-31-3
- ^ Kuhn Richard (1933). "Molekulare asymmetrie". Stereochemie (Kark Freudenberg, Ed.). Leipzig-Wien:Franz-Deutike. pp. 803–824.
- ^ Christie, George Hallatt; Kenner, James (1 January 1922). "LXXI. The molecular configurations of polynuclear aromatic compounds. Part I. The resolution of 6:6'-dinitro- and 4:6 :4':6'-tetranitro-diphenic acids into optically active components". Journal of the Chemical Society, Transactions. 121: 614–620. doi:10.1039/CT9222100614.
- ^ Ōki, Michinori (1983) Recent Advances in Atropisomerism, in Topics in Stereochemistry, Vol. 14 (N. L. Allinger, E. L. Eliel and S. H. Wilen, Eds.), Hoboken, NJ:John Wiley & Sons, pp. 1-82; published online in 2007, DOI: 10.1002/9780470147238.ch1, see [1] and [2], accessed 12 June 2014.
- ^ a b c d Alkorta, Ibon; Jose Elguero; Christian Roussel; Nicolas Vanthuyne; Patrick Piras (2012). "Atropisomerism and Axial Chirality in Heteroaromatic Compounds". Advances in Heterocyclic Chemistry. 105: 1–188. doi:10.1016/B978-0-12-396530-1.00001-2.
- ^ LaPlante, Steven R.; Edwards, Paul J.; Fader, Lee D.; Jakalian, Araz; Hucke, Oliver (7 March 2011). "Revealing Atropisomer Axial Chirality in Drug Discovery". ChemMedChem. 6 (3): 505–513. doi:10.1002/cmdc.201000485.
- ^ Cepanec, Ivica (2004). Synthesis of biaryls (1st ed.). Amsterdam: Elsevier. ISBN 0080444121.
- ^ Clayden, Jonathan (2004). "Atropisomers and near-atropisomers: achieving stereoselectivity by exploiting the conformational preferences of aromatic amides". Chemical Communications: 127–135. doi:10.1039/b307976g.
- ^ Relaying Asymmetry of Transient Atropisomers of o-Iodoanilides by Radical Cyclizations Marc Petit, Andre J. B. Lapierre, and Dennis P. Curran J. Am. Chem. Soc.; 2005; 127(43) pp 14994 - 14995; (Communication) doi:10.1021/ja055666d Abstract
- ^ Cozzi, Pier Giorgio; Enrico Emer; Andrea Gualandi (2011). "Atroposelective Organocatalysis". Angew. Chem. Int. Ed. 50: 3847–3849. doi:10.1002/anie.201008031.
- ^ Cardoso, Flavio S. P.; Abboud, Khalil A.; Aponick, Aaron (2 October 2013). "Design, Preparation, and Implementation of an Imidazole-Based Chiral Biaryl P,N-Ligand for Asymmetric Catalysis". Journal of the American Chemical Society. 135 (39): 14548–14551. doi:10.1021/ja407689a.
- ^ Fukuyama, Yoshiyasu; Asakawa, Yoshinori (1991). "Novel neurotrophic isocuparane-type sesquiterpene dimers, mastigophorenes A, B, C and D, isolated from the liverwort Mastigophora diclados". Journal of the Chemical Society, Perkin Transactions 1 (11): 2737. doi:10.1039/p19910002737.
- ^ a b Zask, Arie; John Murphy; George A Ellestad (2013). "Biological Stereoselectivity of Atropisomeric Natural Products and Drugs". Chirality. 25: 265–274. doi:10.1002/chir.22145.
- ^ Joncour, A; Décor A; Thoret S; Chiaroni A; Baudoin O. (2006). "Biaryl axis as a stereochemical relay for the enantioselective synthesis of antimicrotubule agents". Angew. Chem. Int. Ed. 45: 4149–4152. doi:10.1002/anie.200600451.
- ^ a b Clayden, J.; Moran, W. J.; Edwards, P. J.; LaPante, S. R. (2009). "The Challenge of Atropisomerism in Drug Discovery". Angew. Chem. Int. Ed. 48: 6398–6401. doi:10.1002/anie.200901719. PMID 19637174.



