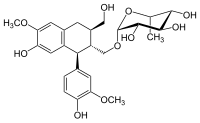
Aviculin
Appearance
| |
| Names | |
|---|---|
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C26H34O10 | |
| Molar mass | 506.54 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Aviculin is a lignan. It is bio-active isolate of Pseudocydonia sinensis[1] or Polygonum aviculare.[2]
References
- ^ Gao, H; Wu, L; Kuroyanagi, M; et al. (November 2003). "Antitumor-promoting constituents from Chaenomeles sinensis KOEHNE and their activities in JB6 mouse epidermal cells". Chem. Pharm. Bull. 51: 1318–21. doi:10.1248/cpb.51.1318. PMID 14600382.
{{cite journal}}: Explicit use of et al. in:|last4=(help) - ^ Ja Kim, Hyoung (1994). "A Novel Lignan and Flavonoids from Polygonum aviculare". Journal of Natural Products. 57: 581–586. doi:10.1021/np50107a003.
