Bacteriorhodopsin
Bacteriorhodopsin is a protein used by Archaea, most notably by Halobacteria, a class of the Euryarchaeota.[1] It acts as a proton pump; that is, it captures light energy and uses it to move protons across the membrane out of the cell.[2] The resulting proton gradient is subsequently converted into chemical energy.[3]
Structure

Bacteriorhodopsin is an integral membrane protein usually found in two-dimensional crystalline patches known as "purple membrane", which can occupy up to nearly 50% of the surface area of the archaeal cell. The repeating element of the hexagonal lattice is composed of three identical protein chains, each rotated by 120 degrees relative to the others. Each chain has seven transmembrane alpha helices and contains one molecule of retinal buried deep within, the typical structure for retinylidene proteins.
Bacteriorhodopsin is a light-driven proton pump
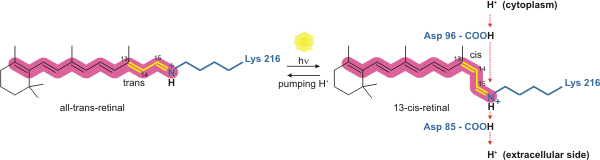
It is the retinal molecule that changes its conformation when absorbing a photon, resulting in a conformational change of the surrounding protein and the proton pumping action.[4] It is covalently linked to Lys216 in the chromophore by Schiff base action. After photoisomerization of the retinal molecule, Asp85 becomes a proton acceptor of the donor proton from the retinal molecule. This releases a proton from a "holding site" into the extracellular side (EC) of the membrane. Reprotonation of the retinal molecule by Asp96 restores its original isomerized form. This results in a second proton being released to the EC side. Asp85 releases its proton into the "holding site," where a new cycle may begin.

The bacteriorhodopsin molecule is purple and is most efficient at absorbing green light (wavelength 500-650 nm, with the absorption maximum at 568 nm). Bacteriorhodopsin has a broad excitation spectrum. For a detection wavelength between 700 and 800 nm, it has an appreciable detected emission for excitation wavelengths between 470 nm and 650 nm (with a peak at 570 nm).[7] When pumped at 633 nm, the emission spectrum has appreciable intensity between 650 nm and 850 nm.[8]
Bacteriorhodopsin belongs to the microbial rhodopsins. They have similarities to vertebrate rhodopsins, the pigments that sense light in the retina. Rhodopsins also contain retinal; however, the functions of rhodopsin and bacteriorhodopsin are different, and there is limited similarity in their amino acid sequences. Both rhodopsin and bacteriorhodopsin belong to the 7TM receptor family of proteins, but rhodopsin is a G protein-coupled receptor and bacteriorhodopsin is not. In the first use of electron crystallography to obtain an atomic-level protein structure, the structure of bacteriorhodopsin was resolved in 1990. It was then used as a template to build models of G protein-coupled receptors before crystallographic structures were also available for these proteins.
Many molecules have homology to bacteriorhodopsin, including the light-driven chloride pump halorhodopsin (for which the crystal structure is also known), and some directly light-activated channels like channelrhodopsin.
All other phototrophic systems in bacteria, algae, and plants use chlorophylls or bacteriochlorophylls rather than bacteriorhodopsin. These also produce a proton gradient, but in a quite different and more indirect way involving an electron transfer chain consisting of several other proteins. Furthermore, chlorophylls are aided in capturing light energy by other pigments known as "antennas"; these are not present in bacteriorhodopsin-based systems. It is possible that phototrophy independently evolved at least twice, once in bacteria and once in archaea.
Gallery
-
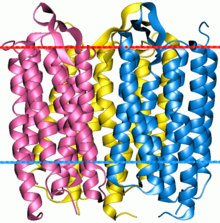
Bacteriorhodopsin is a trimer. Red line indicates extracellular side (EC) of the membrane
-
Bacteriorhodopsin single monomer with retinal molecule between 7 vertical alpha helixes (PDB ID: 1X0S [9][10][11]). One more small helix is light blue, beta sheet yellow.
See also
Literature
- ^ See the NCBI webpage on Halobacteria
- ^ Voet, Judith G.; Voet, Donald (2004). Biochemistry. New York: J. Wiley & Sons. ISBN 0-471-19350-X.
- ^ "Bacteriorhodopsin: Pumping Ions".
- ^ Hayashi S, Tajkhorshid E, Schulten K (September 2003). "Molecular dynamics simulation of bacteriorhodopsin's photoisomerization using ab initio forces for the excited chromophore". Biophysical Journal. 85 (3): 1440–9. doi:10.1016/S0006-3495(03)74576-7. PMC 1303320. PMID 12944261.
- ^ Nicholls D. G.; Ferguson S. J. (1992). Bioenergetics 2 (2nd ed.). San Diego: Academic Press. ISBN 9780125181242.
- ^ Stryer, Lubert (1995). Biochemistry (fourth ed.). New York - Basingstoke: W. H. Freeman and Company. ISBN 978-0716720096.
- ^ Schenkl, Selma; Zgrablic, Goran; Portuondo-Campa, Erwin; Haacke, Stefan; Chergui, Majed (2007). "On the excitation wavelength dependence of the fluorescence of bacteriorhodopsin". Chemical Physics Letters. 441: 322–326. doi:10.1016/j.cplett.2007.04.086.
- ^ Ohtani, H.; Tsukamoto, Y.; Sakoda, Y.; Hamaguchi, H. (1995). "Fluorescence spectra of bacteriorhodopsin and the intermediates O and Q at room temperature". FEBS Lett. 359 (1): 65–68. doi:10.1016/0014-5793(94)01440-c. PMID 7851532.
- ^ a b Nishikawa, T.; Murakami, M. (2005-03-28). "Crystal structure of the 13-cis isomer of bacteriorhodopsin". RCSB Protein Data Bank (PDB). doi:10.2210/pdb1x0s/pdb. PDB ID: 1X0S. Retrieved 7 October 2012.
- ^ a b Nishikawa, T.; Murakami, M. (2005). "Crystal structure of the 13-cis isomer of bacteriorhodopsin in the dark-adapted state". J.Mol.Biol. 352. Elsevier: 319–328. doi:10.1016/j.jmb.2005.07.021. PMID 16084526. PDB ID: 1X0S. Retrieved 7 October 2012.
- ^ a b Image created with RasTop (Molecular Visualization Software).
External links
- Bacteriorhodopsin: Molecule of the Month, by David Goodsell, RCSB Protein Data Bank
- UMich Orientation of Proteins in Membranes families/superfamily-6 - Calculated spatial positions of bacteriorhodopsin-like proteins in membrane

![Bacteriorhodopsin single monomer with retinal molecule between 7 vertical alpha helixes (PDB ID: 1X0S [9][10][11]). One more small helix is light blue, beta sheet yellow.](http://upload.wikimedia.org/wikipedia/commons/2/21/Bacteriorhodopsin_subunit_1X0S.png)
![Bacteriorhodopsin trimer with one retinal molecule in each subunit seen from the extracellular side EC (PDB ID: 1X0S [9][10][11]).](http://upload.wikimedia.org/wikipedia/commons/c/c4/Bacteriorhodopsin_trimer_1X0S.png)