Betulin
| |
| Names | |
|---|---|
| IUPAC name
Lup-20(29)-ene-3β,28-diol
| |
| Other names
betulinol, betuline, betulol, betulinic alcohol, trochol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.797 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H50O2 | |
| Molar mass | 442.728 g·mol−1 |
| Appearance | solid with needle-like crystals[1] |
| Melting point | 256 to 257 °C (493 to 495 °F; 529 to 530 K) |
| insoluble[1] | |
| Solubility | slightly soluble in ethanol and benzene; soluble in diethyl ether, ethyl acetate and ligroin[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Betulin is an abundant, naturally occurring triterpene. It is commonly isolated from the bark of birch trees. It forms up to 30% of the dry weight of silver birch bark.[2] It is also found in birch sap.[citation needed] Inonotus obliquus [3] and red alder also contain betulin.[4]
The compound in the bark gives the tree its white color which appears to protect the tree from mid-winter overheating by the sun. As a result, birches are some of the northern most occurring deciduous trees. It can be converted to betulinic acid (the alcohol group replaced by a carboxylic acid group), which is biologically more active than betulin itself.[citation needed]
History
Betulin was discovered in 1788 by German-Russian chemist Johann Tobias Lowitz.[5][6]
Chemistry
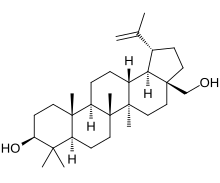
Chemically, betulin is a triterpenoid of lupane structure. It has a pentacyclic ring structure, and hydroxyl groups in positions C3 and C28.
Biological activities
A clinical study has shown that betulin was effective against a variety of tumors. Betulin causes some types of tumor cells to start a process of self-destruction called apoptosis, and can slow the growth of several types of tumor cells.[7]
Another study has shown that betulin inhibited the maturation of sterol regulatory element-binding protein (SREBPs). Inhibition of SREBP by betulin decreased the biosynthesis of cholesterol and fatty acids. In vivo, betulin ameliorated diet-induced obesity, decreased the lipid contents in serum and tissues, and increased insulin sensitivity. Furthermore, betulin reduced the size and improved the stability of atherosclerotic plaques.[8]
Native Americans used red alder bark to treat poison oak, insect bites, and skin irritations. Blackfoot Indians used an infusion made from the bark of red alder to treat lymphatic diseases and tuberculosis.[4]
See also
References
- ^ a b c Haynes, William M.; Lide, David R.; Bruno, Thomas J. (2014). "3". CRC Handbook of Chemistry and Physics (95th ed.). Boca Raton, Florida: CRC Press. p. 340. ISBN 9781482208689. OCLC 908078665.
- ^ Green, Brian; Bentley, Michael D.; Chung, Bong Y.; Lynch, Nicholas G.; Jensen, Bruce L. (2007-12-01). "Isolation of Betulin and Rearrangement to Allobetulin. A Biomimetic Natural Product Synthesis". Journal of Chemical Education. 84 (12). doi:10.1021/ed084p1985.
- ^ Gao, Yuan; Xu, Hongyu; Lu, Zhenming; Xu, Zhenghong (November 2009). "Quantitative determination of steroids in the fruiting bodies and submerged-cultured mycelia of Inonotus obliquus". Se Pu = Chinese Journal of Chromatography. 27 (6): 745–749. ISSN 1000-8713. PMID 20352924.
- ^ a b Gregory L., Tilford (1997). Edible and medicinal plants of the West. Missoula, Mont.: Mountain Press Pub. p. 12. ISBN 9780878423590. LCCN 97000842. OCLC 36307825.
- ^ Lowitz, J. T. (1788). "Űber eine neue, fast benzoeartige substanz der briken". Crell’s Chem. Ann. 1: 312–317.
- ^ Król, Sylwia Katarzyna; Kiełbus, Michał; Rivero-Müller, Adolfo; Stepulak, Andrzej (2015). "Comprehensive Review on Betulin as a Potent Anticancer Agent". BioMed Research International. 2015. doi:10.1155/2015/584189. PMC 4383233. PMID 25866796.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Alakurtti, Sami; Mäkelä, Taru; Koskimies, Salme; Yli-Kauhaluoma, Jari (September 2006). "Pharmacological properties of the ubiquitous natural product betulin". European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences. 29 (1): 1–13. doi:10.1016/j.ejps.2006.04.006. ISSN 0928-0987. PMID 16716572.
- ^ Tang, Jing-Jie; Li, Jia-Gui; Qi, Wei; Qiu, Wen-Wei; Li, Pei-Shan; Li, Bo-Liang; Song, Bao-Liang. "Inhibition of SREBP by a Small Molecule, Betulin, Improves Hyperlipidemia and Insulin Resistance and Reduces Atherosclerotic Plaques". Cell Metabolism. 13 (1): 44–56. doi:10.1016/j.cmet.2010.12.004. PMID 21195348.
