Bipyridine
Bipyridines also known as bipyridyls, dipyridyls, and dipyridines,[1] are a family of chemical compounds with the formula (C5H4N)2, which are formed by the coupling of two pyridine rings. They are aromatic nitrogen heterocycles that form complexes with most transition metals. They interact with metals via both σ-donating nitrogen atoms and π-accepting molecular orbitals. Bipyridines figure prominently in studies of electron and energy transfer, supramolecular and materials chemistry, and catalysis.
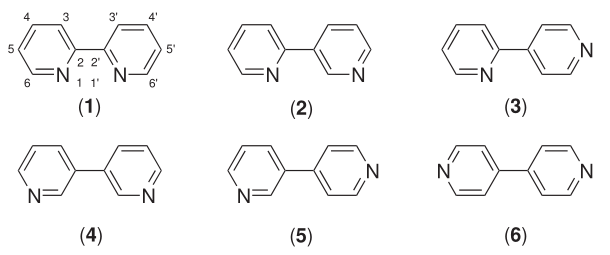
Six isomers of bipyridine exist, but two isomers are prominent: 2,2'-bipyridine is a popular ligand in coordination chemistry and 4,4'-bipyridine is a precursor to the herbicide paraquat. The bipyridines are colourless solids, which are soluble in organic solvents and slightly soluble in water.
The 3,4'-Bipyridine derivatives inamrinone and milrinone are used occasionally for short term treatment of congestive heart failure. They inhibit phosphodiesterase and thus increasing cAMP, exerting positive inotropy and causing vasodilation. Inamrinone causes thrombocytopenia. Milrinone decreases survival in heart failure.
2,2'-bipyridine

2,2'-bipyridine is a chelating ligand that forms complexes with most transition metal ions. Many of these complexes have distinctive optical properties and some are of interest for analysis. 2,2'-Bipyridine is used in the manufacture of Diquat.
4,4'-bipyridine

4,4'-Bipyridine (4,4'-bipy) is mainly used as a precursor to the N,N'-dimethyl-4,4'-bipyridinium dication commonly known as paraquat. This species is redox active, and its toxicity arises from its ability to interrupt biological electron transfer processes. Because of its structure, 4,4'-bipyridine can bridge between metal centres to give coordination polymers.
