Bisphenol
The bisphenols (pronounced /ˈbɪs.fɪ.nɒl/) are a group of chemical compounds with two hydroxyphenyl functionalities. Most of them are based on diphenylmethane. The exceptions are bisphenol S, P, and M. "Bisphenol" is a common name; the letter following refers to one of the reactants. Bisphenol A is the most popular representative of this group, often simply called "bisphenol."
List
| Structural formula | Name | CAS | Reactants | Systematic name | |
|---|---|---|---|---|---|
| Bisphenol A | 80-05-7 | Phenol | Acetone | 2,2-Bis(4-hydroxyphenyl)propane | |
 |
Bisphenol AP | 1571-75-1 | Phenol | Acetophenone | 1,1-Bis(4-hydroxyphenyl)-1-phenyl-ethane |
 |
Bisphenol AF | 1478-61-1 | Phenol | Hexafluoroacetone | 2,2-Bis(4-hydroxyphenyl)hexafluoropropane |
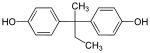 |
Bisphenol B | 77-40-7 | Phenol | Butanone | 2,2-Bis(4-hydroxyphenyl)butane |
 |
Bisphenol BP | 1844-01-5 | Phenol | Benzophenone | Bis-(4-hydroxyphenyl)diphenylmethane |
 |
Bisphenol C | 79-97-0 | Cresol | Acetone | 2,2-Bis(3-methyl-4-hydroxyphenyl)propane |
 |
Bisphenol C 2 | 14868-03-2 | Phenol | Dichloromethane | Bis(4-hydroxyphenyl)-2,2-dichlorethylene |
| Bisphenol E | 2081-08-5 | Phenol | Acetaldehyde | 1,1-Bis(4-hydroxyphenyl)ethane | |
| Bisphenol F | 87139-40-0 | Phenol | Formaldehyde | Bis(4-hydroxyphenyl)methane | |
 |
Bisphenol G | 127-54-8 | 2-Isopropylphenol | Acetone | 2,2-Bis(4-hydroxy-3-isopropyl-phenyl)propane |
| Bisphenol M | 13595-25-0 | 1,3-Bis(2-(4-hydroxyphenyl)-2-propyl)benzene | |||
 |
Bisphenol S | 80-09-1 | Phenol | Sulfur trioxide | Bis(4-hydroxyphenyl)sulfone |
 |
Bisphenol P | 2167-51-3 | 1,4-Bis(2-(4-hydroxyphenyl)-2-propyl)benzene | ||
 |
Bisphenol PH | 24038-68-4 | 2-Phenylphenol | Acetone | 5,5’ -(1-Methylethyliden)-bis[1,1’-(bisphenyl)-2-ol]propane |
 |
Bisphenol TMC | 129188-99-4 | Phenol | 3,3,5-Trimethylcyclohexanone | 1,1-Bis(4-hydroyphenyl)-3,3,5-trimethyl-cyclohexane |
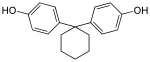 |
Bisphenol Z | 843-55-0 | Phenol | Cyclohexanone | 1,1-Bis(4-hydroxyphenyl)-cyclohexane |
Bisphenol A diglycidyl ether (BADGE) is another bisphenol derivative, along with EPI-001.
Health effects
Bisphenols A (BPA) and S (BPS) have been shown to be endocrine disruptors.[1][2] Due to its high production volumes BPA has been characterised as a "pseudo-persistent" chemical,[3] leading to its spreading and potential accumulation in a variety of environmental matrices.
Prevention measures
These products tend to release from the material when heated; as a precaution, it is recommended for the consumer:
- not to heat food in a plastic packaging in a microwave oven, or a tin can (its inside coating is often epoxy) with a bain-marie; [citation needed]
- use a pitcher material other than plastic, not a plastic bottle.[citation needed]
References
- ^ "BPA-Free Plastic Containers May Be Just as Hazardous". Scientific American. Retrieved 8 August 2015.
- ^ "Bisphenol A (BPA) & Bisphenol S (BPS)". SaferChemicals.org. Retrieved 8 August 2015.
- ^ Pivnenko, K.; Pedersen, G. A.; Eriksson, E.; Astrup, T. F. (2015-10-01). "Bisphenol A and its structural analogues in household waste paper". Waste Management. 44: 39–47. doi:10.1016/j.wasman.2015.07.017. PMID 26194879.
