Branches of physics

Physics deals with the combination of matter and energy. It also deals with a wide variety of systems, about which theories have been developed that are used by physicists. In general, theories are experimentally tested numerous times before they are accepted as correct as a description of Nature (within a certain domain of validity). For instance, the theory of classical mechanics accurately describes the motion of objects, provided they are much larger than atoms and moving at much less than the speed of light. These theories continue to be areas of active research: for instance, a remarkable aspect of classical mechanics known as chaos was discovered in the 20th century, three centuries after the original formulation of classical mechanics by Isaac Newton (1642–1727). These "central theories" are important tools for research in more specialized topics, and any physicist, regardless of his or her specialization, is expected to be literate in them.
Classical mechanics
Classical mechanics is a model of the physics of forces acting upon bodies. It is often referred to as "Newtonian mechanics" after Isaac Newton and his laws of motion. It also includes classical approach as given by Hamiltonian and Lagrange methods. It deals with motion of particles and general system of particles.
Thermodynamics and statistical mechanics
The first chapter of The Feynman Lectures on Physics is about the existence of atoms, which Feynman considered to be the most compact statement of physics, from which science could easily result even if all other knowledge was lost.[1] By modeling matter as collections of hard spheres, it is possible to describe the kinetic theory of gases, upon which classical thermodynamics is based.
Thermodynamics studies the effects of changes in temperature, pressure, and volume on physical systems on the macroscopic scale, and the transfer of energy as heat.[2][3] Historically, thermodynamics developed out of the desire to increase the efficiency of early steam engines.[4]
The starting point for most thermodynamic considerations is the laws of thermodynamics, which postulate that energy can be exchanged between physical systems as heat or work.[5] They also postulate the existence of a quantity named entropy, which can be defined for any system.[6] In thermodynamics, interactions between large ensembles of objects are studied and categorized. Central to this are the concepts of system and surroundings. A system is composed of particles, whose average motions define its properties, which in turn are related to one another through equations of state. Properties can be combined to express internal energy and thermodynamic potentials, which are useful for determining conditions for equilibrium and spontaneous processes.
Electromagnetism
| Maxwell's equations of electromagnetism |
Relativity
The special theory of relativity enjoys a relationship with electromagnetism and mechanics; that is, the principle of relativity and the principle of stationary action in mechanics can be used to derive Maxwell's equations,[7][8] and vice versa.
The theory of special relativity was proposed in 1905 by Albert Einstein in his article "On the Electrodynamics of Moving Bodies". The title of the article refers to the fact that special relativity resolves an inconsistency between Maxwell's equations and classical mechanics. The theory is based on two postulates: (1) that the mathematical forms of the laws of physics are invariant in all inertial systems; and (2) that the speed of light in a vacuum is constant and independent of the source or observer. Reconciling the two postulates requires a unification of space and time into the frame-dependent concept of spacetime.
General relativity is the geometrical theory of gravitation published by Albert Einstein in 1915/16.[9][10] It unifies special relativity, Newton's law of universal gravitation, and the insight that gravitation can be described by the curvature of space and time. In general relativity, the curvature of spacetime is produced by the energy of matter and radiation.
Quantum mechanics

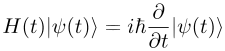
Quantum mechanics is the branch of physics treating atomic and subatomic systems and their interaction with radiation. It is based on the observation that all forms of energy are released in discrete units or bundles called "quanta". Remarkably, quantum theory typically permits only probable or statistical calculation of the observed features of subatomic particles, understood in terms of wave functions. The Schrödinger equation plays the role in quantum mechanics that Newton's laws and conservation of energy serve in classical mechanics—i.e., it predicts the future behavior of a dynamic system—and is a wave equation that is used to solve for wavefunctions.
For example, the light, or electromagnetic radiation emitted or absorbed by an atom has only certain frequencies (or wavelengths), as can be seen from the line spectrum associated with the chemical element represented by that atom. The quantum theory shows that those frequencies correspond to definite energies of the light quanta, or photons, and result from the fact that the electrons of the atom can have only certain allowed energy values, or levels; when an electron changes from one allowed level to another, a quantum of energy is emitted or absorbed whose frequency is directly proportional to the energy difference between the two levels. The photoelectric effect further confirmed the quantization of light.
In 1924, Louis de Broglie proposed that not only do light waves sometimes exhibit particle-like properties, but particles may also exhibit wave-like properties. Two different formulations of quantum mechanics were presented following de Broglie's suggestion. The wave mechanics of Erwin Schrödinger (1926) involves the use of a mathematical entity, the wave function, which is related to the probability of finding a particle at a given point in space. The matrix mechanics of Werner Heisenberg (1925) makes no mention of wave functions or similar concepts but was shown to be mathematically equivalent to Schrödinger's theory. A particularly important discovery of the quantum theory is the uncertainty principle, enunciated by Heisenberg in 1927, which places an absolute theoretical limit on the accuracy of certain measurements; as a result, the assumption by earlier scientists that the physical state of a system could be measured exactly and used to predict future states had to be abandoned. Quantum mechanics was combined with the theory of relativity in the formulation of Paul Dirac. Other developments include quantum statistics, quantum electrodynamics, concerned with interactions between charged particles and electromagnetic fields; and its generalization, quantum field theory.
Interdisciplinary fields
To the interdisciplinary fields, which define partially sciences of their own, belong e.g. the
- astrophysics, the physics in the universe, including the properties and interactions of celestial bodies in astronomy.
- biophysics, studying the physical interactions of biological processes.
- chemical physics, the science of physical relations in chemistry.
- econophysics, dealing with physical processes and their relations in the science of economy.
- geophysics, the sciences of physical relations on our planet.
- medical physics, the application of physics to prevention, diagnosis, and treatment.
- physical chemistry, dealing with physical processes and their relations in the science of physical chemistry.
Summary
The table below lists the core theories along with many of the concepts they employ.
References
- ^ Feynman, Richard Phillips; Leighton, Robert Benjamin; Sands, Matthew Linzee (1963). The Feynman Lectures on Physics. p. 1-1. ISBN 0-201-02116-1.. Feynman begins with the atomic hypothesis, as his most compact statement of all scientific knowledge: "If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations ..., what statement would contain the most information in the fewest words? I believe it is ... that all things are made up of atoms – little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another. ..." vol. I p. I–2
- ^ Perot, Pierre (1998). A to Z of Thermodynamics. Oxford University Press. ISBN 0-19-856552-6.
- ^ Clark, John O.E. (2004). The Essential Dictionary of Science. Barnes & Noble Books. ISBN 0-7607-4616-8.
- ^ Clausius, Ruldolf (1850). "LXXIX". On the Motive Power of Heat, and on the Laws which can be deduced from it for the Theory of Heat. Dover Reprint. ISBN 0-486-59065-8.
{{cite book}}:|work=ignored (help) - ^ Van Ness, H.C. (1969). Understanding Thermodynamics. Dover Publications, Inc. ISBN 0-486-63277-6.
- ^ Dugdale, J. S. (1998). Entropy and its Physical Meaning. Taylor and Francis. ISBN 0-7484-0569-0.
- ^ Landau and Lifshitz (1951, 1962), The Classical Theory of Fields, Library of Congress Card Number 62-9181, Chapters 1–4 (3rd edition is ISBN 0-08-016019-0)
- ^ Corson and Lorrain, Electromagnetic Fields and Waves ISBN 0-7167-1823-5
- ^ Einstein, Albert (November 25, 1915). "Die Feldgleichungen der Gravitation". Sitzungsberichte der Preussischen Akademie der Wissenschaften zu Berlin: 844–847. Retrieved 2006-09-12.
- ^ Einstein, Albert (1916). "The Foundation of the General Theory of Relativity". Annalen der Physik. Bibcode:1916AnP...354..769E. doi:10.1002/andp.19163540702. Archived from the original (PDF) on 2006-08-29. Retrieved 2006-09-03.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)

