Bridging ligand
A bridging ligand is a ligand that connects two or more atoms, usually metal ions.[1] The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals.
In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek character 'mu', μ[2], with a superscript number denoting the number of metals bound to the bridging ligand is bound. μ2 is often denoted simply as μ.

Illustrative bridging ligands
Virtually all ligands are known to bridge, with the exception of amines and ammonia.[3] Particularly common inorganic bridging ligands include
Cyanide usually bridges via M-NC-M' linkages, unlike the other entries on this list.
Many organic ligands form strong bridges between metal centers. Many common examples include derivatives of the above inorganic ligands (R = alkyl, aryl):
- OR−,
- SR−,
- NR2−
- NR2− (imido)
- P3− (phosphido)
- PR2− (phosphido, note the ambiguity with the preceding entry)
- PR2− (phosphinidino)
Polyfunctional ligands
Polyfunctional ligands can attach to metals in many ways and thus can bridge metals in diverse ways, including sharing of one atom or using several atoms. Examples of such polyatomic ligands are the oxoanions CO32− and the related Carboxylate, PO43−, and the polyoxometallates. Several organophosphorus ligands have been developed that bridge pairs of metals, a well-known example being Ph2PCH2PPh2.
Examples
| Compound | Formula | Description |
|---|---|---|
 |
{(Fe(III)(OH2)4)2(µ-OH)2}4+ | In this example hydroxide plays the role of a μ2 bridging ligand. Notice in the name of the compound μ2 has been simplified to μ.[4] |
 |
(η6-C6H6)2Ru2Cl2(μ-Cl)2 | In this particular complex, two chloride ligands are terminal and two are μ2 bridging. The η in the beginning of the formula denotes the hapticity of the benzene ligands. |
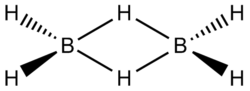 |
B2H6 | This classic borane compound, diborane features two μ2 bridging hydrides. |
 |
(Co(CO)3)3(μ3-(C-tBu)) | This compound contains a μ3 bridging carbyne ligand (C-tBu). |
See Also
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "bridging ligand". doi:10.1351/goldbook.B00741
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "µ- (mu)". doi:10.1351/goldbook.B00741
- ^ Werner, H. (2004). "The Way into the Bridge: A New Bonding Mode of Tertiary Phosphanes, Arsanes, and Stibanes". Angew. Chem. Int. Ed. 43 (8): 938–954. doi:10.1002/anie.200300627. PMID 14966876.
- ^ Classifications of ligands. http://chimge.unil.ch/En/complexes/1cpx7.htm
