Bromethalin
This article needs additional citations for verification. (October 2016) |
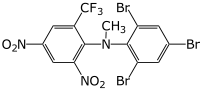
| |
| Names | |
|---|---|
| IUPAC name
N-Methyl-2,4-dinitro-6-(trifluoromethyl)-N-(2',4',6'-tribromophenyl)aniline
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.109.042 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H7Br3F3N3O4 | |
| Molar mass | 577.93 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bromethalin is a rodenticide which poisons the central nervous system by uncoupling mitochondrial oxidative phosphorylation, which causes a decrease in adenosine triphosphate (ATP) synthesis. Decreased ATP ultimately results in increased intracranial pressure, which damages neuronal axons. This damage to the central nervous system can cause paralysis, convulsions, and death.[1]
Risk of poisoning
No tests will diagnose bromethalin poisoning in pets, but signs to watch for include severe muscle tremors, hyperexcitability, fits, extreme sensitivity to being touched (hyperesthesia) and seizures that appear to be caused by light or noise. Signs can be delayed for several days.
No antidote for bromethalin is known; care is symptomatic and supportive. Owners of animals that have eaten it accidentally should seek immediate veterinary attention and be decontaminated. Contacting an animal poison control center can help ensure that timely and appropriate therapy is started.
The mechanism of bromethalin toxicity differs from that of popular rodent poisons, which are anticoagulants related to warfarin (Coumadin). These drugs, such as diphacinone and bromadiolone, eaten by the mouse as an overdose, inhibit vitamin K and lead to a loss of clotting activity over several days (clotting factors require vitamin K). The ultimate result is hemorrhage.
While bromethalin is labeled as a rodenticide, it is often used for other small mammals such as moles. It is often hidden inside a worm-like bait to attract moles.
References
- ^ Bromethalin, Animal Disease Diagnostic Laboratory, Spring 1997 Newsletter
