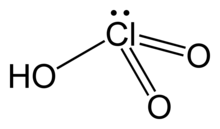
Chloric acid
| |

| |
| Names | |
|---|---|
| Other names
Chloric(V) acid
| |
| Identifiers | |
| ECHA InfoCard | 100.029.303 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| HClO3 | |
| Molar mass | 84.45914 g mol−1 |
| Appearance | colourless solution |
| Density | 1 g/mL, solution (approximate) |
| >40 g/100 ml (20 °C) | |
| Acidity (pKa) | ca. −1 |
| Structure | |
| pyramidal | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Oxidant |
| Related compounds | |
Other anions
|
bromic acid iodic acid |
Other cations
|
ammonium chlorate sodium chlorate potassium chlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chloric acid, HClO3, is an oxoacid of chlorine, and the formal precursor of chlorate salts. It is a strong acid (pKa ≈ −1) and oxidizing agent.
It is prepared by the reaction of sulfuric acid with barium chlorate, the insoluble barium sulfate being removed by precipitation:
- Ba(ClO3)2 + H2SO4 → 2HClO3 + BaSO4
Another method is the heating of hypochlorous acid, of which productions include chloric acid and hydrogen chloride:
- 3HClO → HClO3 + 2 HCl
It is stable in cold aqueous solution up to a concentration of approximately 30%, and solution of up to 40% can be prepared by careful evaporation under reduced pressure. Above these concentrations, and on warming, chloric acid solutions decompose to give a variety of products, for example:
- 8HClO3 → 4HClO4 + 2H2O + 2Cl2 + 3 O2
- 3HClO3 → HClO4 + H2O + 2 ClO2
The decomposition is controlled by kinetic factors: indeed, chloric acid is never thermodynamically stable with respect to disproportionation.
See also
References
This article has an unclear citation style. (September 2007) |
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- King, R. B. (Ed.) (1994) Encyclopedia of Inorganic Chemistry, Vol. 2, p. 658. Chichester:Wiley. ISBN 0-471-93620-0
