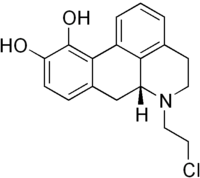
Chloroethylnorapomorphine
Appearance
| |
| Names | |
|---|---|
| IUPAC name
6-(2-Chloro-ethyl)-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-10,11-diol
| |
| Other names
(−)-N-(2-Chloroethyl)-norapomorphine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H18ClNO2 | |
| Molar mass | 315.80 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chloroethylnorapomorphine is a chemical once thought to be an irreversible dopamine D2 receptor antagonist;[1][2] however, it was later proved to be reversible.[3]
References
- ^ Cohen, SA; Neumeyer, JL. (Oct 1983). "Kinetics of solvolysis of N-(2-chloroethyl)norapomorphine, an irreversible dopamine receptor antagonist". Journal of Medicinal Chemistry. 26 (10): 1348–53. doi:10.1021/jm00364a003. PMID 6620296.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Guan, JH; Neumeyer, JL; Filer, CN; Ahern, DG; Lilly, L; Watanabe, M; Grigoriadis, D; Seeman, P. (Jun 1984). "N-(2-chloroethyl) [8,9-2H]norapomorphine, an irreversible ligand for dopamine receptors: synthesis and application". Journal of Medicinal Chemistry. 27 (6): 806–10. doi:10.1021/jm00372a019. PMID 6737423.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Lehmann, J; Langer, SZ (1982). "Dopamine autoreceptors differ pharmacologically from postsynaptic dopamine receptors: Effects of (−)-N-(2-chloroethyl)-norapomorphine". European Journal of Pharmacology. 77 (1): 85–6. doi:10.1016/0014-2999(82)90542-8. PMID 7060630.
