Chloroprene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Chlorobuta-1,3-diene
| |||
| Other names
Chloroprene, 2-chloro-1,3-butadiene, Chlorobutadiene, β-Chloroprene
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.381 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H5Cl | |||
| Molar mass | 88.5365 g/mol | ||
| Appearance | Colorless liquid | ||
| Odor | pungent, ether-like | ||
| Density | 0.9598 g/cm3 | ||
| Melting point | −130 °C (−202 °F; 143 K) | ||
| Boiling point | 59.4 °C (138.9 °F; 332.5 K) | ||
| 0.026 g/100 mL | |||
| Solubility | soluble in alcohol, diethyl ether miscible in ethyl ether, acetone, benzene | ||
| Vapor pressure | 188 mmHg (20 °C)[1] | ||
Refractive index (nD)
|
1.4583 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Highly flammable, toxic. | ||
| NFPA 704 (fire diamond) | |||
| Flash point | −15.6 °C (3.9 °F; 257.5 K) | ||
| Explosive limits | 1.9%–11.3%[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
450 mg/kg (rat, oral) | ||
LC50 (median concentration)
|
3207 ppm (rat, 4 hr)[2] | ||
LCLo (lowest published)
|
1052 ppm (rabbit, 8 hr) 350 ppm (cat, 8 hr)[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 25 ppm (90 mg/m3) [skin][1] | ||
REL (Recommended)
|
Ca C 1 ppm (3.6 mg/m3) [15-minute][1] | ||
IDLH (Immediate danger)
|
300 ppm[1] | ||
| Related compounds | |||
Related Dienes
|
Butadiene Isoprene | ||
Related compounds
|
Vinyl chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Chloroprene is the common name for the organic compound 2-chlorobuta-1,3-diene, which has the formula CH2=CCl−CH=CH2. This colorless liquid is the monomer for the production of the polymer polychloroprene, a type of synthetic rubber. Polychloroprene is better known to the public as Neoprene, the trade name given by DuPont.
Production of chloroprene
Chloroprene is produced in three steps from 1,3-butadiene: (i) chlorination, (ii) isomerization of part of the product stream, and (iii) dehydrochlorination of 3,4-dichlorobut-1-ene.
Chlorine adds to 1,3-butadiene to afford a mixture of 3,4-dichlorobut-1-ene and 1,4-dichlorobut-2-ene. The 1,4-dichloro isomer is subsequently isomerized to 3,4 isomer, which in turn is treated with base to induce dehydrochlorination to 2-chlorobuta-1,3-diene. This dehydrohalogenation entails loss of a hydrogen atom in the 3 position and the chlorine atom in the 4 position thereby forming a double bond between carbons 3 and 4. In 1983, approximately 2,000,000 kg was produced in this manner.[3] The chief impurity in chloroprene prepared in this way is 1-chlorobuta-1,3-diene, which is usually separated by distillation.
Acetylene process
Until the 1960s, chloroprene production was dominated by the "acetylene process," which was modeled after the original synthesis of vinylacetylene.[4] In this process, acetylene is dimerized to give vinyl acetylene, which is then combined with hydrogen chloride to afford 4-chloro-1,2-butadiene (an allene derivative), which in the presence of copper(I) chloride, rearranges to the targeted 2-chlorobuta-1,3-diene:[3]
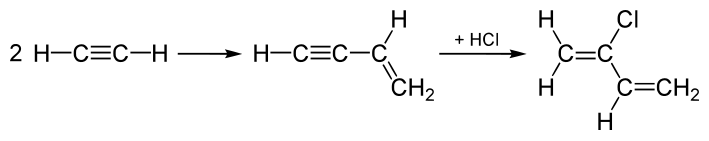
This process is very energy-intensive and has high investment costs. Furthermore, the intermediate vinyl acetylene is unstable.
This "acetylene process" has been replaced by a process which adds Cl2 to one of the double bonds in 1,3-butadiene instead, and subsequent elimination produces HCl instead, as well as chloroprene.
Transportation regulations
Transportation of uninhibited chloroprene has been banned in the United States by the US Department of Transportation. Stabilized chloroprene is in hazard class 3 (flammable liquid). Its UN number is 1991 and is in packing group 1.
References
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0133". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "ß-Chloroprene". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Manfred Rossberg, Wilhelm Lendle, Gerhard Pfleiderer, Adolf Tögel, Eberhard-Ludwig Dreher, Ernst Langer, Heinz Rassaerts, Peter Kleinschmidt, Heinz Strack, Richard Cook, Uwe Beck, Karl-August Lipper, Theodore R. Torkelson, Eckhard Löser, Klaus K. Beutel, "Chlorinated Hydrocarbons" in Ullmann’s Encyclopedia of Industrial Chemistry, 2006 John Wiley-VCH: Weinheim. doi:10.1002/14356007.a06_233.pub2
- ^ Wallace H. Carothers, Ira Williams, Arnold M. Collins, and James E. Kirby (1937). "Acetylene Polymers and their Derivatives. II. A New Synthetic Rubber: Chloroprene and its Polymers". J. Am. Chem. Soc. 53 (11): 4203–4225. doi:10.1021/ja01362a042.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- International Chemical Safety Card 0133
- NIOSH Pocket Guide to Chemical Hazards. "#0133". National Institute for Occupational Safety and Health (NIOSH).
- IARC Monograph "Chloroprene."



