Cholecalciferol
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
(3β,5Z,7E)-9,10-secocholesta-
5,7,10(19)-trien-3-ol | |||
| Other names
vitamin D3, activated 7-dehydrocholesterol.
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.612 | ||
| EC Number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C27H44O | |||
| Molar mass | 384.64 g/mol | ||
| Appearance | White, needle-like crystals | ||
| Melting point | 83–86 °C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Cholecalciferol is a form of vitamin D, also called vitamin D3 or calciol.[1][2]
It is structurally similar to steroids such as testosterone, cholesterol, and cortisol (though vitamin D3 itself is a secosteroid).
Forms
Vitamin D3 has several forms:
- Cholecalciferol, (sometimes called calciol) is an inactive, unhydroxylated form of vitamin D3)
- Calcifediol (also called calcidiol, hydroxycholecalciferol, or 25-hydroxyvitamin D3, and abbreviated 25(OH)D) is one of the forms measured in the blood to assess vitamin D status[3]
- Calcitriol (also called 1,25-dihydroxyvitamin D3) is the active form of D3.
Metabolism
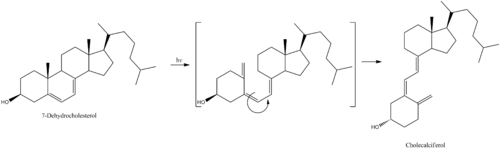
7-Dehydrocholesterol is the precursor of vitamin D3 and forms cholecalciferol only after being exposed to solar UV radiation and also indoor sun tanning machines.
Cholecalciferol is then hydroxylated in the liver to become calcifediol (25-hydroxyvitamin D3).
Next, calcifediol is again hydroxylated, this time in the kidney, and becomes calcitriol (1,25-dihydroxyvitamin D3). Calcitriol is the most active hormone form of vitamin D3.
Regulation of metabolism
- Cholecalciferol is synthesized in the skin from 7-dehydrocholesterol under the action of ultraviolet B light. It reaches an equilibrium after several minutes depending on several factors including conditions of sunlight (latitude, season, cloud cover, altitude), age of skin, and color of skin.
- Hydroxylation in the endoplasmic reticulum of liver hepatocytes of cholecalciferol to calcifediol (25-hydroxycholecalciferol) by 25-hydroxylase is loosely regulated, if at all, and blood levels of this molecule largely reflect the amount of vitamin D3 produced in the skin or the vitamin D2 or D3 ingested.
- Hydroxylation in the kidneys of calcifediol to calcitriol by 1-alpha-hydroxylase is tightly regulated (stimulated by either parathyroid hormone or hypophosphatemia) and serves as the major control point in production of the most active circulating hormone calcitriol (1,25-dihydroxyvitamin D3).
As food fortification
Although cholecalciferol can be synthesized in the skin (see Metabolism), it is also a form of vitamin D added to fortify foods. Cholecalciferol is produced industrially by the irradiation of 7-dehydrocholesterol extracted from lanolin found in sheep's wool. In foods where animal products are not desired, an alternative compound is ergocalciferol (also known as vitamin D2) derived from the fungal sterol ergosterol.
Dose
One gram of pure vitamin D3 is 40 000 000 (40x106) IU, where one IU is equivalent to 0.025 μg. Recommendations are: 15 micrograms (600 IU or International Units) daily for all individuals (males, female, pregnant/lactating women) under the age of 70 years-old. For all individuals older than 70 years, 20 micrograms daily (800 IU) is recommended.[4] A growing body of researchers question whether the current recommended adequate levels are sufficient to meet physiological needs, particularly for individuals deprived of regular sun exposure or those at higher risk such as those with higher-melanin content in the skin (i.e. those whose ancestors are African, Latin American, or Asian), the obese, and those who live far from the equator. The upper limit (UL) for vitamin D has been recommended as 4,000 IU daily. The 4,000 IU cut-off was determined by the Institute of Medicine in 2010 after reviewing the then-current medical literature, finding that toxicity had consistently occurred when doses of 40,000 IU daily were taken,[5] and that there was a single case of toxicity above 10,000 IU daily; this case of toxicity occurred under circumstances which have led other researchers to dispute it as a credible case to consider when making vitamin D intake recommendations.[5] The Institute of Medicine did not find evidence of toxicity between 4,000 IU and 10,000 IU, so the 4,000 IU number is more of an estimate than a number based on evidence of toxicity above 4,000 IU.[4] Patients with severe vitamin D deficiency will require treatment with a loading dose, its magnitude can be calculated based on the actual serum 25-hydroxy-vitamin D level and body weight.[6]
Also, there is a therapy for rickets utilizing a single dose, called stoss therapy in Europe - taking from 300 000 IU (7500 μg) to 500 000 IU (12 500 μg), as a single dose, or two to four divided doses.[7]
The 25-hydroxy vitamin D (calcifediol) blood test is used to determine how much vitamin D is in the body. The normal range of calcifediol is 30.0 to 74.0 ng/mL.[3]
"Vitamin D toxicity can result from regular excess intake of this vitamin, and may lead to hypercalcemia and excess bone loss. Individuals at particular risk include those with hyperparathyroidism, kidney disease, sarcoidosis, tuberculosis, or histoplasmosis. Chronic hypercalcemia may lead to serious or even life-threatening complications, and should be managed by a physician. Early symptoms of hypercalcemia may include nausea, vomiting, and anorexia (appetite/weight loss), followed by polyuria (excess urination), polydipsia (excess thirst), weakness, fatigue, somnolence, headache, dry mouth, metallic taste, vertigo, tinnitus (ear ringing), and ataxia (unsteadiness). Kidney function may become impaired, and metastatic calcifications (calcium deposition in organs throughout the body) may occur, particularly affecting the kidneys. Treatment involves stopping the intake of vitamin D or calcium, and lowering the calcium levels under strict medical supervision, with frequent monitoring of calcium levels. Acidification of urine and corticosteroids may be necessary."[8]
There are conflicting reports concerning the absorption of cholecalciferol (D3) versus ergocalciferol (D2), with some studies suggesting less efficacy of D2,[9] and others showing no difference.[10] At present, D2 and D3 doses are frequently considered interchangeable, but more research is needed to clarify this.
Stability
Cholecalciferol is very sensitive to UV radiation and will rapidly, but reversibly, break down to form supra-sterols, which can further irreversibly convert to ergosterol.
Preventative application
A 2008 study published in Cancer Research has shown the addition of vitamin D3 (along with calcium) to the diet of some mice fed a regimen similar in nutritional content to a new Western diet with 1000 IU cholecaliferol per day prevented colon cancer development.[11] In humans, with 400 IU daily, there was no effect.[12]
Toxicity
At high doses cholecalciferol is poisonous. Rodents are somewhat more susceptible to high doses than other species, and cholecalciferol has been used in poison bait for the control of these pests. It has been claimed that the compound is less toxic to non-target species. However, in practice it has been found that use of cholecalciferol in rodenticides represents a significant hazard to other animals, such as dogs and cats. "Cholecalciferol produces hypercalcemia, which results in systemic calcification of soft tissue, leading to renal failure, cardiac abnormalities, hypertension, CNS depression, and GI upset. Signs generally develop within 18-36 hr of ingestion and can include depression, anorexia, polyuria, and polydipsia."[13]
In New Zealand, possums have become a significant pest animal, and cholecalciferol has been used as the active ingredient in lethal gel baits for possum control. The LD50 is 16.8 mg/kg, but only 9.8 mg/kg if calcium carbonate is added to the bait.[14][15]
Kidneys and heart are target organs.[16]
See also
- Hypervitaminosis D, Vitamin D poisoning
- Ergocalciferol, vitamin D2.
- 25-Hydroxyvitamin D3 1-alpha-Hydroxylase, a kidney enzyme that converts calcifediol to calcitriol.
References
- ^ "Nomenclature of Vitamin D. Recommendations 1981. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN)" reproduced at the Queen Mary, University of London website. Retrieved 21 March 2010.
- ^ "cholecalciferol" at Dorland's Medical Dictionary
- ^ a b ""25-hydroxy vitamin D test: Medline Plus". Retrieved 21 March 2010.
- ^ a b DRIs for Calcium and Vitamin D
- ^ a b Vieth R (1999). "Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety". Am. J. Clin. Nutr. 69 (5): 842–56. PMID 10232622.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ van Groningen L, Opdenoordt S, van Sorge A, Telting D, Giesen A, de Boer H (2010). "Cholecalciferol loading dose guideline for vitamin D-deficient adults". Eur. J. Endocrinol. 162 (4): 805–11. doi:10.1530/EJE-09-0932. PMID 20139241.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Shah BR, Finberg L (1994). "Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method". J. Pediatr. 125 (3): 487–90. doi:10.1016/S0022-3476(05)83303-7. PMID 8071764.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Vitamin D: MedlinePlus Supplements with the National Academy of Sciences dosing recommendations at the Medline Plus website.
- ^ Armas LA, Hollis BW, Heaney RP (2004). "Vitamin D2 is much less effective than vitamin D3 in humans". J. Clin. Endocrinol. Metab. 89 (11): 5387–91. doi:10.1210/jc.2004-0360. PMID 15531486.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD (2008). "Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D". J. Clin. Endocrinol. Metab. 93 (3): 677–81. doi:10.1210/jc.2007-2308. PMC 2266966. PMID 18089691.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, Corner G, Livote E, Lesser M, Edelmann W, Velcich A, Lipkin M, Augenlicht L (2008). "Dietary induction of colonic tumors in a mouse model of sporadic colon cancer". Cancer Res. 68 (19): 7803–10. doi:10.1158/0008-5472.CAN-08-1209. PMID 18829535.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE (2006). "Calcium plus vitamin D supplementation and the risk of colorectal cancer". N. Engl. J. Med. 354 (7): 684–96. doi:10.1056/NEJMoa055222. PMID 16481636.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Merck Veterinary Manual - Rodenticide Poisoning: Introduction".
- ^ Morgan D (2006). "Field efficacy of cholecalciferol gel baits for possum (Trichosurus vulpecula) control". New Zealand Journal of Zoology. 33 (3): 221–8. doi:10.1080/03014223.2006.9518449.
- ^ Jolly SE, Henderson RJ, Frampton C, Eason CT (1995). "Cholecalciferol Toxicity and Its Enhancement by Calcium Carbonate in the Common Brushtail Possum". Wildlife Research. 22 (5): 579–83. doi:10.1071/WR9950579.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Kiwicare Material Safety Data Sheet" (PDF).