Copper(I) fluoride
| |
| Names | |
|---|---|
| IUPAC name
Copper(I) fluoride
| |
| Systematic IUPAC name
Fluorocopper[1] | |
| Other names
Cuprous fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CuF | |
| Molar mass | 82.544 g·mol−1 |
| Density | 7.1 g cm-3 |
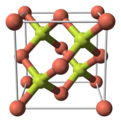
| Structure | |
| sphalerite | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Copper(I) fluoride is an inorganic compound with the chemical formula CuF. Its existence is uncertain. It was reported in 1933 to have a sphalerite-type crystal structure.[2] Modern textbooks state that CuF is not known,[3] since fluorine is so electronegative that it will always oxidise copper to its +2 oxidation state.[4] Complexes of CuF such as [(Ph3P)3CuF] are, however, known and well characterised.[5]
Synthesis and reactivity
It can be formed by the reduction of copper(II) fluoride.[citation needed] Unlike copper(I) chloride, copper(I) fluoride tends to disproportionate into copper(II) fluoride and copper in a one to one ratio at ambient conditions, unless it is stabilised through complexation as in the example of [Cu(N2)F].
- CuF → Cu + CuF2
As a result of this disproportiontion, samples slowly become light cyan in colour, the colour of copper(II) fluoride.[citation needed]
References
- ^ "Copper Monofluoride - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ^ Ebert, F.; Woitinek, H. (1933). "Kristallstrukturen von Fluoriden. II. HgF, HgF2, CuF und CuF2". Z. anorg. allg. Chem. 210 (3): 269–272. doi:10.1002/zaac.19332100307.
- ^ Housecroft, C. E.; Sharpe, A. G. (2008). Inorganic Chemistry (3rd ed.). Prentice Hall. pp. 737–738. ISBN 978-0-13-175553-6.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1183–1185. ISBN 978-0-08-037941-8.
- ^ Gulliver, D. J.; Levason, W.; Webster, M. (1981). "Coordination Stabilised Copper(I) Fluoride. Crystal and Molecular Structure of
Fluorotris(triphenylphosphine)copper(I)·Ethanol (1/2), Cu(PPh3)3F·2EtOH". Inorg. Chim. Acta. 52: 153–159. doi:10.1016/S0020-1693(00)88590-4.
{{cite journal}}: line feed character in|title=at position 80 (help)
