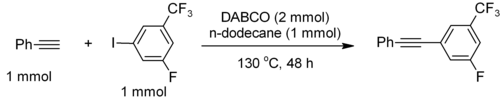DABCO
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,4-diazabicyclo[2.2.2]octane
| |||
| Other names
triethylenediamine, TEDA
DABCO | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.005.455 | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H12N2 | |||
| Molar mass | 112.17 g/mol | ||
| Appearance | White crystalline powder | ||
| Melting point | 156 - 160 °C decomposes | ||
| Boiling point | 174 °C | ||
| Soluble, hygroscopic | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Harmful | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
DABCO or 1,4-diazabicyclo[2.2.2]octane is a chemical compound. It is used as a catalyst, particularly in making polyurethanes, and complexing ligand. DABCO is a useful catalyst in the Baylis-Hillman reaction. While most low-molecular weight amines have strong, disagreeable odors, DABCO smells like peanuts.
DABCO is also used to adjust pH of the oxygen-sensitive resin to regulate the reaction rate in Flexplay time-limited DVDs. Antioxidants, like DABCO, are used to improve the lifetime of dyes. This makes DABCO useful in dye lasers and in mounting samples for fluorescence microscopy (when used with glycerol and PBS).[2] DABCO can also be used to demethylate quaternary ammonium salts by heating in N,N-dimethylformamide (DMF).[3]
DABCO has also been used as a catalyst for a metal-free Sonogashira coupling, with or without microwave enhancement.[4] For example, phenylacetylene couples with electron-deficient iodoarenes to furnish the Sonogashira product in 77% yield with 95% selectivity.
Dabco is a registered trademark for Air Products' amine catalyst product line including 1,4-diazabicyclo[2.2.2]octane.
References
- ^ "Safety data for 1,4-diazabicyclo(2.2.2)octane". Oxford University.
- ^ Valnes K, Brandtzaeg P (1985). "Retardation of immunofluorescence fading during microscopy". J. Histochem. Cytochem. 33 (8): 755–61. PMID 3926864.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Ho, T.L (1972). "Dealkylation of Quaternary Ammonium Salts with 1,4-Diazabicyclo[2.2.2]octane". Synthesis. 1972: 702. doi:10.1055/s-1972-21977.
- ^ Luque, Rafael (2009). "Efficient solvent- and metal-free Sonogashira protocol catalysed by 1,4-diazabicyclo(2.2.2) octane (DABCO)". Organic and Biomolecular Chemistry. 7 (8). Royal Society of Chemistry: 1627–1632. doi:10.1039/b821134p. PMID 19343249.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)