Dioxygen difluoride
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Dioxygen difluoride | |||
| Systematic IUPAC name
Fluorooxy hypofluorite | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | FOOF | ||
| ChEBI | |||
| ChemSpider | |||
| 1570 | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| O 2F 2 | |||
| Molar mass | 69.996 g·mol−1 | ||
| Melting point | −154 °C (−245 °F; 119 K) | ||
| Boiling point | −57 °C (−71 °F; 216 K) | ||
| Solubility in other solvents | decomp. | ||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Dioxygen difluoride is a compound with the formula O
2F
2. It exists as an orange solid that melts into a red liquid at −163 °C.[2] It is a strong oxidant and decomposes into OF
2 and oxygen even at −160 °C (4% per day) - it thus can not exist at room temperature.[3]
Dioxygen difluoride is one of the most unstable and explosive chemicals ever synthesized. It reacts explosively with nearly every chemical it encounters, even ordinary ice, leading to its nickname, FOOF, a play on its chemical formula. Another nickname, coined by chemist Derek Lowe, is Satan's Kimchi.[1]
Preparation
Dioxygen difluoride can be obtained by subjecting a 1:1 mixture of gaseous fluorine and oxygen at low pressure (7–17 mmHg is optimal) to an electric discharge of 25–30 mA at 2.1–2.4 kV. This is basically the reaction used for the first synthesis by Otto Ruff in 1933.[4] Another synthesis involves mixing O2 and F2 in a stainless steel vessel cooled to −196 °C, followed by exposing the elements to 3 MeV bremsstrahlung for several hours. A third method requires heating a mix of Fluorine and Oxygen to 700 °C (1,292 °F), and then rapidly cooling it using liquid oxygen.[5] Fluorine is extremely dangerous and unstable at these temperatures and this synthesis should not be attempted.[1]
Structure and electronic description
In O
2F
2, oxygen is assigned the unusual oxidation state of +1. In most of its other compounds, oxygen has an oxidation state of −2.
The structure of dioxygen difluoride resembles that of hydrogen peroxide, H
2O
2, in its large dihedral angle, which approaches 90°. This geometry conforms with the predictions of VSEPR theory. The O−O bond length is within 2 pm of the 120.7 pm distance for the O=O double bond in dioxygen, O2.
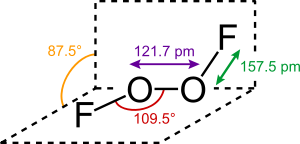
The bonding within dioxygen difluoride has been the subject of considerable speculation over the years, particularly because of the very short O–O distance and the long O–F distances. Bridgeman has proposed a scheme which essentially has an O–O triple bond and an O–F single bond that is destabilised and lengthened by repulsion between the lone pairs on the fluorine atoms and the π-orbitals of the O–O bond.[6] Repulsion involving the fluorine lone pairs is also responsible for the long and weak covalent bonding in the fluorine molecule. The 19F NMR chemical shift of dioxygen difluoride is 865 ppm, which is by far the highest chemical shift recorded for a fluorine nucleus, thus underlining the extraordinary electronic properties of this compound.
Reactivity
The overarching property of this unstable compound is its oxidizing power, despite the fact that all reactions must be conducted near −100 °C.[7] A.G. Streng conducted a series of experiments with the substance to test its reactions. Streng's experiments resulted in a series of fires and explosions. Some of the compounds that produced violent reactions with FOOF include ethyl alcohol, methane, ammonia, and even with water ice.[7] Despite Streng's willingness to react extremely dangerous compounds, he was unable to perform some of the experiments due to their extreme explosiveness. For example, four moles of dioxygen difluoride reacting with one mole of hydrogen sulfide produces 433 kilocalories of energy, which commentator Derek Lowe calls "the kind of every-man-for-himself exotherm that you want to avoid at all cost". Lowe continues, "A. G. Streng, folks, absolutely takes the corrosive exploding cake, and I have to tip my asbestos-lined titanium hat to him."[1]
With BF3 and PF5, it gives the corresponding dioxygenyl salts:[3][8]
- 2 O
2F
2 + 2 PF
5 → 2 [O
2]+
[PF
6]−
+ F
2
Applications
Due to its extreme instability and the low temperatures required for it to exist, there are no practical applications. In his experiments, Streng succesfully stored it in solid form at 90 K (−183.2 °C). Los Alamos National Laboratory has reportedly used it to convert uranium and plutonium oxides into the corresponding hexafluorides[9], but otherwise, the safety problems have prevented this chemical from seeing any use. In 2010, a company called Hangzhou Sage Chemical Company claimed to offer the chemical for sale. It is highly doubtful that they actually have the chemical, due to the problems in both storing and shipping it safely.[1]
See Also
References
- ^ a b c d e Derek Lowe (February 23, 2010), "Things I Won't Work With: Dioxygen Difluoride", In the Pipeline, retrieved April 9, 2013
- ^
Kirshenbaum, A. D.; Grosse, A. V. (1959). "Ozone Fluoride or Trioxygen Difluoride, O
3F
2". Journal of the American Chemical Society. 81 (6): 1277. doi:10.1021/ja01515a003. - ^ a b Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. Academic Press. ISBN 0-12-352651-5.
- ^
Ruff, O.; Mensel, W. (1933). "Neue Sauerstofffluoride: O
2F
2 und OF". Zeitschrift für anorganische und allgemeine Chemie. 211 (1–2): 204–208. doi:10.1002/zaac.19332110122. - ^ Thomas Mills. "Direct synthesis of liquid-phase dioxygen difluoride". Retrieved April 9, 2013.
{{cite journal}}: Cite journal requires|journal=(help)(subscription required) - ^ Bridgeman, A. J.; Rothery, J. (1999). "Bonding in mixed halogen and hydrogen peroxides". Journal of the Chemical Society, Dalton Transactions. 1999 (22): 4077–4082. doi:10.1039/a904968a.
- ^ a b Streng, A. G. (1963). "The Chemical Properties of Dioxygen Difluoride". Journal of the American Chemical Society. 85 (10): 1380–1385. doi:10.1021/ja00893a004.
- ^ Solomon, I. J. (1964). "New Dioxygenyl Compounds". Inorganic Chemistry. 3 (3): 457. doi:10.1021/ic50013a036.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Atwood, D. A. (2006). "Fluorine: Inorganic Chemistry". Encyclopedia of Inorganic Chemistry. John Wiley & Sons. doi:10.1002/0470862106.ia076.