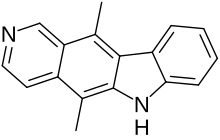
Ellipticine

| ||||
| ||||
| Names | ||||
|---|---|---|---|---|
| IUPAC name
5,11-dimethyl-6H-pyrido[4,3-b]carbazole
| ||||
| Identifiers | ||||
3D model (JSmol)
|
||||
| ChEBI | ||||
| ChemSpider | ||||
| ECHA InfoCard | 100.007.514 | |||
| EC Number |
| |||
| KEGG | ||||
PubChem CID
|
||||
| UNII | ||||
CompTox Dashboard (EPA)
|
||||
| ||||
| ||||
| Properties | ||||
| C17H14N2 | ||||
| Molar mass | 246.313 g/mol[1] | |||
| Appearance | Yellow solid[2] | |||
| Density | 1.257±0.06 g/cm3[3] | |||
| Melting point | 316–318 °C (601–604 °F; 589–591 K)[3] | |||
| Very low[4] | ||||
| Hazards | ||||
| GHS labelling: | ||||
| class="wikitable collapsible" style="min-width: 50em;" | ||||
| Pictogram | Code | Symbol description | Image link | |
 |
GHS01 | {{GHS exploding bomb}} | Image:GHS-pictogram-explos.svg | Explosive |
 |
GHS02 | {{GHS flame}} | Image:GHS-pictogram-flamme.svg | |
 |
GHS03 | {{GHS flame over circle}} | Image:GHS-pictogram-rondflam.svg | |
 |
GHS04 | {{GHS gas cylinder}} | Image:GHS-pictogram-bottle.svg | |
 |
GHS05 | {{GHS corrosion}} | Image:GHS-pictogram-acid.svg | Corrosive |
 |
GHS06 | {{GHS skull and crossbones}} | Image:GHS-pictogram-skull.svg | Accute Toxic |
 |
GHS07 | {{GHS exclamation mark}} | Image:GHS-pictogram-exclam.svg | Irritant |
 |
GHS08 | {{GHS health hazard}} | Image:GHS-pictogram-silhouette.svg | Health Hazard |
 |
GHS09 | {{GHS environment}} | Image:GHS-pictogram-pollu.svg | Environment |
See also
|-
|-
| style="padding-left:1em;" |
| H301[1]
|-
|-
| style="padding-left:1em;" |
| P264, P270, P301+P310, P321, P330, P405, P501[1]
|-
| colspan=2 style="text-align:left; background:#f8eaba; border:1px solid #a2a9b1;" |
|-
|}
Ellipticine is an alkaloid first extracted from trees of the species Ochrosia elliptica and Rauvolfia sandwicensis,[6][7] which inhibits the enzyme topoisomerase II via intercalative binding to DNA.[8]
Natural occurrence and synthesis
Ellipticine is an organic compound present in several trees within the genera Ochrosia, Rauvolfia, Aspidosperma, and Apocynaceae.[9] It was first isolated from Ochrosia elliptica Labill., a flowering tree native to Australia and New Caledonia which gives the alkaloid its name, in 1959,[6] and synthesised by Robert Burns Woodward later the same year.[7]
Biological activity
Ellipticine is a known intercalator, capable of entering a DNA strand between base pairs. In its intercalated state, ellipticine binds strongly[10] and lies parallel to the base pairs,[11] increasing the superhelical density of the DNA.[12] Intercalated ellipticine binds directly to topoisomerase II, an enzyme involved in DNA replication,[13] inhibiting the enzyme and resulting in powerful antitumour activity.[11] In clinical trials, ellipticine derivatives have been observed to induce remission of tumour growth, but are not used for medical purposes due to their high toxicity; side effects include nausea and vomiting, hypertension, cramp, pronounced fatigue, mouth dryness, and mycosis of the tongue and oesophagus.[14]
Further DNA damage results from the formation of covalent DNA adducts following enzymatic activation of ellipticine by with cytochromes P450 and peroxidases, meaning that ellipticine is classified as a prodrug.[15]
References
- ^ a b c d "Ellipticine | C17H14N2 - PubChem". PubChem. 2016. Retrieved 2017-05-30.
- ^ Miller, R B; Dugar, S (1989). "A regiospecific total synthesis of ellipticine via nitrene insertion". Tetrahedron Letters. 30 (3): 297–300. doi:10.1016/S0040-4039(00)95184-0. ISSN 0040-4039.
- ^ a b "Ellipticine | 519-23-3". ChemicalBook. 2016. Retrieved 2017-05-30.
- ^ Sbai, M; Ait Lyazidi, S; Lerner, D A; del Castillo, B; Martin, M A (1996). "Use of micellar media for the fluorimetric determination of ellipticine in aqueous solutions". Journal of Pharmaceutical and Biomedical Analysis. 14 (8): 959–965. doi:10.1016/S0731-7085(96)01759-1. ISSN 0731-7085.
- ^ "Globally Harmonized System of Classification and Labelling of Chemicals" (pdf). 2021. Annex 3: Codification of Statements and Pictograms (pp 268–385).
- ^ a b Goodwin, S; Smith, A F; Horning, E C (1959). "Alkaloids of Ochrosia elliptica Labill". Journal of the American Chemical Society. 81 (8): 1903–1908.
- ^ a b Woodward, R B; Iacobucci, G A; Hochstein, I A (1959). "The synthesis of ellipticine". Journal of the American Chemical Society. 81 (16): 4434–4435. doi:10.1021/ja01525a085. ISSN 0002-7863.
- ^ Auclair, C (1987). "Multimodal action of antitumor agents on DNA: The ellipticine series". Archives of Biochemistry and Biophysics. 259 (1): 1–14. doi:10.1016/0003-9861(87)90463-2. ISSN 0003-9861.
- ^
Isah, T (2016). "Anticancer Alkaloids from Trees: Development into Drugs". Pharmacognosy Reviews. 10 (20): 90–99. doi:10.4103/0973-7847.194047. ISSN 0973-7847. PMC 5214563. PMID 28082790.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kohn, K W; Waring, M J; Glaubiger, D; Friedman, C A (1975). "Intercalative Binding of Ellipticine to DNA". Cancer Research. 35 (1): 71–76. ISSN 0008-5472. PMID 1109798.
- ^ a b Canals, A; Purciolas, M; Aymamí, J; Coll, M (2005). "The anticancer agent ellipticine unwinds DNA by intercalative binding in an orientation parallel to base pairs". Acta Crystallographica D. 61 (7): 1009–1012. doi:10.1107/S0907444905015404. ISSN 0907-4449.
- ^ Chu, Y; Hsu, M T (1992). "Ellipticine increases the superhelical density of intracellular SV40 DNA by intercalation". Nucleic Acids Research. 20 (15): 4033–4038. ISSN 0305-1048. PMC 334084. PMID 1324474.
- ^ Froelich-Ammon, S J; Patchan, M W; Osheroff, N; Thompson, R B (1995). "Topoisomerase II binds to ellipticine in the absence or presence of DNA. Characterization of enzyme-drug interactions by fluorescence spectroscopy". Journal of Biological Chemistry. 270 (25): 14998–15004. ISSN 0021-9258. PMID 7797481.
- ^ Paoletti, C; Le Pecq, J B; Dat-Xuong, N; Juret, P; Garnier, H; Amiel, J L; Rouesse, J (1980). "Antitumor activity, pharmacology, and toxicity of ellipticines, ellipticinium, and 9-hydroxy derivatives: preliminary clinical trials of 2-methyl-9-hydroxy ellipticinium (NSC 264-137)". Recent Results in Cancer Research. 74: 107–123. ISSN 0080-0015. PMID 7003658.
- ^ Stiborová, M; Poljaková, J; Martínková, E; Ulrichová, J; Šimánek, V; Dvořák, Z; Frei, E (2012). "Ellipticine oxidation and DNA adduct formation in human hepatocytes is catalyzed by human cytochromes P450 and enhanced by cytochrome b5". Toxicology. 302 (2–3): 233–241. doi:10.1016/j.tox.2012.08.004. ISSN 0300-483X.
