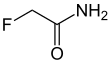
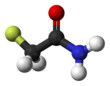
Fluoroacetamide
Appearance
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-fluoroacetamide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.010.331 | ||
| KEGG | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H4FNO | |||
| Molar mass | 77.058 | ||
| Melting point | 107 to 109 °C (225 to 228 °F; 380 to 382 K) | ||
| Soluble | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Fluoroacetamide is an organic compound based on acetamide with one fluorine atom replacing hydrogen on the methyl group. it is a metabolic poison which disrupts the citric acid cycle and was used as a rodenticide.