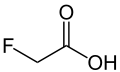
Fluoroacetic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
Fluoroacetic acid | |
| Other names
2-Fluoroacetic acid
Monofluoroacetic acid Monofluoroacetate Fluoroethanoic acid Cymonic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1739053 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.120 |
| EC Number |
|
| 25730 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2642 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H3FO2 | |
| Molar mass | 78.042 g·mol−1 |
| Appearance | White crystals |
| Density | 1.369 |
| Melting point | 35.2 °C (95.4 °F; 308.3 K) |
| Boiling point | 165 °C (329 °F; 438 K) |
| Soluble in water and ethanol | |
| Acidity (pKa) | 2.586 |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H300, H314, H400 | |
| P260, P264, P270, P273, P280, P301+P310, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fluoroacetic acid is a chemical compound with formula CH2FCOOH. The sodium salt, sodium fluoroacetate, is used as a pesticide. It inhibits the aconitase step of the citric acid cycle.[1]
Natural occurrence

Fluoroacetate occurs naturally in at least 40 plants in Australia, Brazil, and Africa. It is one of only five known organic fluorine-containing natural products.[2] It was first identified in Dichapetalum cymosum, commonly known as gifblaar (Afrikaans) or poison leaf, by Marais in 1944.[3][4] As early as 1904, colonists in Sierra Leone used extracts of Chailletia toxicaria, which also contains fluoroacetic acid or its salts, to poison rats.[5][6][7] Several native Australian plant genera contain the toxin, including: Gastrolobium, Gompholobium, Oxylobium, Nemcia, and Acacia.
Fluoroacetate occurrence in Gastrolobium species
Gastrolobium is a genus of flowering plants in the family Fabaceae. There are over 100 species in this genus, and all but two are native to the south west region of Western Australia, where they are known as "poison peas". Gastrolobium growing in south western Australia are unique in their ability to concentrate fluoroacetate from low-fluorine soils.[8] Brush-tailed possums, bush rats, and western grey kangaroos native to this region are capable of safely eating plants containing fluoroacetate, but livestock and introduced species from elsewhere in Australia are highly susceptible to the poison,[9] as are species introduced from outside Australia, such as the red fox. The fact that many Gastrolobium species also have high secondary toxicity to non-native carnivores is thought to have limited the ability of cats to establish populations in locations where the plants form a major part of the understorey vegetation.[10]
The presence of Gastrolobium species in the fields of farmers in Western Australia has often forced these farmers to 'scalp' their land, that is remove the top soil and any poison pea seed which it may contain, and replace it with a new poison pea-free top soil sourced from elsewhere in which to sow crops. Similarly, after bushfires in north-western Queensland, cattlemen have to move livestock before the poisonous Gastrolobium grandiflorum emerges from the ashes.[11]
See also
References
- ^ Proudfoot, A. T.; Bradberry, S. M.; Vale, J. A. (2006). "Sodium fluoroacetate poisoning". Toxicology Reviews. 25 (4): 213–219. doi:10.2165/00139709-200625040-00002. PMID 17288493.
- ^ K.K. Jason Chan; David O'Hagan (2012). "The Rare Fluorinated Natural Products and Biotechnological Prospects for Fluorine Enzymology". Methods in Enzymology. 516: 219–235.
- ^ Marais, J. C. S. (1943). "The isolation of the toxic principle "K cymonate" from "Gifblaar", Dichapetalum cymosum". Onderstepoort Journal of Veterinary Science and Animal Industry. 18: 203.
- ^ Marais, J. C. S. (1944). "Monofluoroacetic acid, the toxic principle of "gifblaar" Dichapetalum cymosum". Onderstepoort Journal of Veterinary Science and Animal Industry. 20: 67.
- ^ Renner (1904). "Chemical and Physiological Examination of the Fruit of Chailletia Toxicaria". Jour African Soc.: 109.
- ^ Power, F. B.; Tutin, F. (1906). "Chemical and Physiological Examination of the Fruit of Chailletia toxicaria". Journal of the American Chemical Society. 28 (9): 1170–1183. doi:10.1021/ja01975a007.
- ^ Vartiainen, T.; Kauranen, P. (1984). "The determination of traces of fluoroacetic acid by extractive alkylation, pentafluorobenzylation and capillary gas chromatography-mass spectrometry". Analytica Chimica Acta. 157 (1): 91–97. doi:10.1016/S0003-2670(00)83609-0.
- ^ Lee, J. (1998). "Deadly plants face threat of extinction". ANU Reporter. 29 (6). Australian National University. Retrieved 2012-08-07.
- ^ McKenzie, R. (1997). "Australian Native Poisonous Plants". Australian Plants Online. Australian Native Plants Society. Retrieved 2012-08-07.
- ^ Short, J.; Atkins, L.; Turner, B. (2005). Diagnosis of Mammal Decline in Western Australia, with Particular Emphasis on the Possible Role of Feral Cats and Poison Peas (pdf). Australia: Wildlife Research and Management Pty. Retrieved 2011-09-26.
- ^ Noble group
