Gassman indole synthesis
The Gassman indole synthesis is a series of chemical reactions used to synthesize substituted indoles from aniline.

This is a one-pot chemical reaction, and none of the intermediates are isolated. R1 can be hydrogen or alkyl, while R2 works best with aryl, but can also be alkyl. Electron-rich anilines, such as 4-methoxyaniline, tend to fail in this reaction.
The 3-position thiomethyl group is often removed using Raney nickel to give the 3-H-indole.
Reaction mechanism
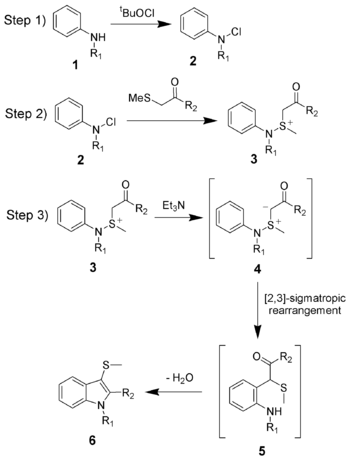
The reaction mechanism of the Gassman indole synthesis is divided among three steps.
The first step is the oxidation of the aniline 1 using tert-butyl hypochlorite (tBuOCl) to give the chloramine 2.
The second step is the addition of the ketone to give the sulfonium ion 3, and is typically done at low temperatures (-78 °C).
The third and final step is the addition of a base, which in this case is triethylamine. Upon warming to room temperature, the base will deprotonate the sulfonium ion creating the sulfonium ylide 4, which quickly undergoes a [2,3]-sigmatropic rearrangement to give the ketone 5. The ketone 5 will undergo a facile condensation to give the desired 3-thiomethylindole 6.
References
- Gassman, P. G.; Gruetzmacher, G.; van Bergen, T. J. J. Am. Chem. Soc., 1973, 95, 6508.
- Gassman, P. G.; van Bergen, T. J.; Gilbert, D. P.; Cue, Jr., B. W. J. Am. Chem. Soc., 1974, 96, 5495.
- Gassman, P. G.; van Bergen, T. J. J. Am. Chem. Soc., 1974, 96, 5508.
- Gassman, P. G.; Gruetzmacher, G.; van Bergen, T. J. J. Am. Chem. Soc., 1974, 96, 5512.
- Organic Syntheses, Coll. Vol. 6, p.601; Vol. 56, p.72 (Article)
