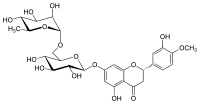
Hesperidin
| |
| Names | |
|---|---|
| IUPAC name
(2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxy-2,3-dihydrochromen-4-one
| |
| Other names
Hesperetin 7-rutinoside[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.536 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H34O15 | |
| Molar mass | 610.565 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hesperidin is a flavanone glycoside found in citrus fruits. Its aglycone form is called hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.
Hesperidin was first isolated in 1828 by French chemist Lebreton from the white inner layer of citrus peels (mesocarp, albedo).[2]
Hesperidin is believed to play a role in plant defense.
Sources
- in Rutaceae
- 700 - 2,500 ppm in fruit of Citrus aurantium L. - Bitter Orange, Petitgrain[3]
- in orange juice (Citrus sinensis)
- in Zanthoxylum gilletii[4]
- in lemon[5]
- in lime[5]
- in leaves of Agathosma serratifolia
- in Lamiaceae
Peppermint also contains hesperidin.[6]
Metabolism
Hesperidin 6-O-alpha-L-rhamnosyl-beta-D-glucosidase is an enzyme that uses hesperidin and H2O to produce hesperetin and rutinose. It is found in the hyphomycetes species Stilbella fimetaria.
Research
As a flavanone found in citrus fruits (such as oranges, lemons or pummelo fruits), hesperidin is under laboratory research for possible biological properties.[7][8][9] One area of research is focused on the possible chemopreventive effects of hesperidin,[10] but there is no current proof that hesperidin has this role in human cancer mechanisms.
See also
- Diosmin
- List of phytochemicals in food
- List of MeSH codes (D03)
- List of food additives
- List of antioxidants in food
References
- ^ Inderjit, Dakshini, K. M. (1991). "Hesperetin 7-rutinoside (hesperidin) and taxifolin 3-arabinoside as germination and growth inhibitors in soils associated with the weed, Pluchea lanceolata (DC) C.B. Clarke (Asteraceae)". J Chem Ecol. 17 (8): 1585–91. doi:10.1007/BF00984690. PMID 24257882.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lebreton (1828). Journal de Pharmacie et de sciences accessories. 14: 377ff.
{{cite journal}}: Missing or empty|title=(help) - ^ "Citrus aurantium L." Dr. Duke's Phytochemical and Ethnobotanical Databases. 6 Oct 2014.
- ^ Tringali, C.; Spatafora, C.; Calì, V.; Simmonds, M. S. (2001). "Antifeedant constituents from Fagara macrophylla". Fitoterapia. 72 (5): 538–43. doi:10.1016/S0367-326X(01)00265-9. PMID 11429249.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Peterson, J. J.; Beecher, G. R.; Bhagwat, S. A.; Dwyer, J. T.; Gebhardt, S. E.; Haytowitz, D. B.; Holden, J. M. (2006). "Flavanones in grapefruit, lemons, and limes: A compilation and review of the data from the analytical literature" (PDF). Journal of Food Composition and Analysis. 19 (Supplement): S74–S80. doi:10.1016/j.jfca.2005.12.009.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dolzhenko, Y.; Bertea, C. M.; Occhipinti, A.; Bossi, S.; Maffei, M. E. (2010). "UV-B modulates the interplay between terpenoids and flavonoids in peppermint (Mentha × piperita L.)". Journal of Photochemistry and Photobiology B: Biology. 100 (2): 67–75. doi:10.1016/j.jphotobiol.2010.05.003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/jf8006568, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/jf8006568instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/jf204452y, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/jf204452yinstead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.lfs.2014.07.029, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.lfs.2014.07.029instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1155/2012/516981, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1155/2012/516981instead.
