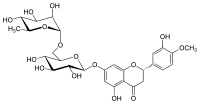
Hesperidin
| |
| Names | |
|---|---|
| IUPAC name
(2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-{[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl}oxan-2-yl]oxy-2,3-dihydrochromen-4-one
| |
| Other names
Hesperetin 7-rutinoside[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.536 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H34O15 | |
| Molar mass | 610.565 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hesperidin is a flavan-on glycoside found in citrus fruits. Its aglycone form is called hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.
Hesperidin was first isolated in 1828 by French chemist Lebreton from the white inner layer of citrus peels (mesocarp, albedo).[2][3]
Hesperidin is believed to play a role in plant defense.
Sources
Rutaceae
- 700 - 2,500 ppm in fruit of Citrus aurantium L. - Bitter Orange, Petitgrain[4]
- in orange juice (Citrus sinensis)
- in Zanthoxylum gilletii[5]
- in lemon[6]
- in lime[6]
- in leaves of Agathosma serratifolia
Lamiaceae
Peppermint contains hesperidin.[7]

Content in foods
Approximate hesperidin content per 100 grams[8]
- 481 mg peppermint, dried
- 44 mg blood orange, pure juice
- 26 mg orange, pure juice
- 18 mg lemon, pure juice
- 14 mg lime, pure juice
- 1 mg grapefruit, pure juice
Metabolism
Hesperidin 6-O-alpha-L-rhamnosyl-beta-D-glucosidase, an enzyme that uses hesperidin and H2O to produce hesperetin and rutinose, is found in the Ascomycetes species.[9]
Research
As a flavanone found in citrus fruits (such as oranges, lemons or pummelo fruits), hesperidin is under laboratory research for possible biological properties.[10][11][12] One area of research is focused on the possible chemopreventive effects of hesperidin,[13] but there is no current proof that hesperidin has this role in human cancer mechanisms.
See also
- Diosmin
- List of phytochemicals in food
- List of MeSH codes (D03)
- List of food additives
- List of antioxidants in food
References
- ^ Inderjit, Dakshini KM (August 1991). "Hesperetin 7-rutinoside (hesperidin) and taxifolin 3-arabinoside as germination and growth inhibitors in soils associated with the weed,Pluchea lanceolata (DC) C.B. Clarke (Asteraceae)". Journal of Chemical Ecology. 17 (8): 1585–91. doi:10.1007/BF00984690. PMID 24257882.
- ^ Lebreton, M (1828). "Sur la matiere cristalline des orangettes, et analyse de ces fruits non encore developpes, famille des Hesperidees". Journal de Pharmacie et de Sciences Accessories. 14: 377ff.
- ^ "Metabocard for Hesperidin (HMDB03265)". Human Metabolome Database, The Metabolomics Innovation Centre, Genome Canada. 11 February 2016. Retrieved 30 October 2016.
- ^ "Citrus aurantium L." Dr. Duke's Phytochemical and Ethnobotanical Databases. 6 Oct 2014. Archived from the original on 2004-11-10.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Tringali, C.; Spatafora, C.; Calì, V.; Simmonds, M. S. (2001). "Antifeedant constituents from Fagara macrophylla". Fitoterapia. 72 (5): 538–43. doi:10.1016/S0367-326X(01)00265-9. PMID 11429249.
- ^ a b Peterson, J. J.; Beecher, G. R.; Bhagwat, S. A.; Dwyer, J. T.; Gebhardt, S. E.; Haytowitz, D. B.; Holden, J. M. (2006). "Flavanones in grapefruit, lemons, and limes: A compilation and review of the data from the analytical literature" (PDF). Journal of Food Composition and Analysis. 19 (Supplement): S74–S80. doi:10.1016/j.jfca.2005.12.009.
- ^ Dolzhenko, Y.; Bertea, C. M.; Occhipinti, A.; Bossi, S.; Maffei, M. E. (2010). "UV-B modulates the interplay between terpenoids and flavonoids in peppermint (Mentha × piperita L.)". Journal of Photochemistry and Photobiology B: Biology. 100 (2): 67–75. doi:10.1016/j.jphotobiol.2010.05.003.
- ^ "Foods in which hesperidin is found". Phenol-Explorer database, version 3.6. Retrieved 15 March 2017.
- ^ Mazzaferro, L; Piñuel, L; Minig, M; Breccia, J. D. (2010). "Extracellular monoenzyme deglycosylation system of 7-O-linked flavonoid beta-rutinosides and its disaccharide transglycosylation activity from Stilbella fimetaria". Archives of Microbiology. 192 (5): 383–93. doi:10.1007/s00203-010-0567-7. PMID 20358178.
- ^ Benavente-García, O.; Castillo, J. (2008). "Update on Uses and Properties of Citrus Flavonoids: New Findings in Anticancer, Cardiovascular, and Anti-inflammatory Activity". Journal of Agricultural and Food Chemistry. 56 (15): 6185–205. doi:10.1021/jf8006568. PMID 18593176.
- ^ Hwang, S. L.; Shih, P. H.; Yen, G. C. (2012). "Neuroprotective Effects of Citrus Flavonoids". Journal of Agricultural and Food Chemistry. 60 (4): 877–85. doi:10.1021/jf204452y. PMID 22224368.
- ^ Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. (2014). "Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin — A mini-review". Life Sciences. 113 (1–2): 1–6. doi:10.1016/j.lfs.2014.07.029. PMID 25109791.
- ^ Tanaka, T.; Tanaka, T.; Tanaka, M.; Kuno, T. (2012). "Cancer Chemoprevention by Citrus Pulp and Juices Containing High Amounts of β-Cryptoxanthin and Hesperidin". Journal of Biomedicine and Biotechnology. 2012: 1–10. doi:10.1155/2012/516981. PMC 3228311. PMID 22174562.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
 Media related to Hesperidin at Wikimedia Commons
Media related to Hesperidin at Wikimedia Commons
