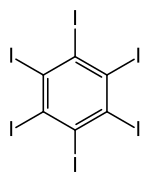
Hexaiodobenzene
Appearance
You can help expand this article with text translated from the corresponding article in German. (September 2014) Click [show] for important translation instructions.
|
| |
| Names | |
|---|---|
| IUPAC name
Hexaiodobenzene
| |
| Other names
Periodobenzene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.009.246 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6I6 | |
| Molar mass | 833.493 g·mol−1 |
| Appearance | orange crystals[1] |
| Density | 4.60 g/cm3 |
| Melting point | 430 °C (806 °F; 703 K)[1] |
| insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hexaiodobenzene is a chemical compound with the formula C6I6. Structurally, it is a derivative of benzene, in which all hydrogen atoms are replaced by iodine atoms. It forms orange crystals[1] that are poorly soluble in all solvents.
The compound was first prepared by iodination of benzoic acid in the presence of hot fuming sulfuric acid.[2] It adopts the expected structure with a central C6 ring.[3]
References
- ^ a b c Daniell Lewis Mattern: Periodination of Benzene with Periodate/Iodide, J. Org. Chem., 1983, 48 (24), pp. 4772–4773 (doi:10.1021/jo00172a063; PDF).
- ^ Erwin Rupp "Ueber die perhalogenirten Phtalsäuren und das Hexajodbenzol", Chem. Ber., 1896, Volume 29, pp. 1625–1634 (doi:10.1002/cber.18960290293).
- ^ Ghosh, Sandip; Reddy, C. Malla; Desiraju, Gautam R. "Hexaiodobenzene: a redetermination at 100 K", Acta Crystallographica, Section E: Structure Reports Online, 2007, 63(2), o910–o911 (doi:10.1107/S1600536807002279).
