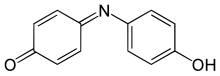
Indophenol
| |
| |

| |
| Names | |
|---|---|
| IUPAC name
4-(4-hydroxyphenyl)iminocyclohexa-2,5-dien-1-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.194 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H9NO2 | |
| Molar mass | 199.209 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Indophenol is an organic compound with the formula OC6H4NC6H4OH. It is deep blue dye that is the product of the Berthelot's reaction, a common test for ammonia.[1] The indophenol group, with various substituents in place of OH and various ring substitutions, is found in many dyes used in hair coloring and textiles.[2]
Berthelot test
In the Berthelot test (1859), a sample suspected of containing ammonia is treated with sodium hypochlorite and phenol. The formation of indophenol is used to determine ammonia and paracetamol by spectrophotometry.[3] Other phenols can be used. Dichlorophenol-indophenol (DCPIP), a form of indophenol, is often used to determine the presence of vitamin C, or ascorbic acid.[4]
See also
Indophenol blue (CAS 132-31-0) is a different compound with systematic name N-(p-dimethylaminophenyl)-1,4-naphthoquinoneimine.[5]
References
- ^ Patton, Charles J.; Crouch, S. R. "Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia" Analytical Chemistry 1977, volume 49, 464-9. Template:DO
- ^ Horst Berneth "Azine Dyes" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_213.pub2
- ^ Tsuboi, T.; Hirano, Y.; Shibata, Y.; Motomizu, S.: Sensitivity Improvement of Ammonia Determination Based on Flow-Injection Indophenol Spectrophotometry with Manganese(II) Ion as a Catalyst and Analysis of Exhaust Gas of Thermal Power Plant, Analytical Sciences, October 2002, Vol. 18, pp. 1141/4
- ^ David Emlyn Hughes "Titrimetric determination of ascorbic acid with 2,6-dichlorophenol indophenol in commercial liquid diets" Journal of Pharmaceutical Sciences 1983, Volume 72, pages 126–129. doi:10.1002/jps.2600720208
- ^ Systematic name of idophenol blue
