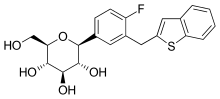
Ipragliflozin
Appearance
 | |
| Clinical data | |
|---|---|
| Trade names | Suglat |
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H21FO5S |
| Molar mass | 404.45 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ipragliflozin (Suglat) is a pharmaceutical drug for treatment of type 2 diabetes. It was approved for use in Japan in 2014.[1]
Ipragliflozin is an SGLT2 inhibitor.[2]
References
- ^ "Ipragliflozin (Suglat) First of New Diabetes Drug Class in Japan". Medscape. January 20, 2014.
- ^ Takasu T, Takakura S, Kaku S (2015). "Pharmacological and clinical profile of ipragliflozin (Suglat(®)): a new therapeutic agent for type 2 diabetes". Nihon Yakurigaku Zasshi. 145 (1): 36–42. doi:10.1254/fpj.145.36. PMID 25743234.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
