Isocyanic acid
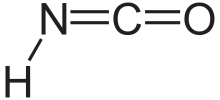
| |

| |
| Names | |
|---|---|
| IUPAC name
Isocyanic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.109.068 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HNCO | |
| Molar mass | 43.03 g/mol |
| Appearance | Colorless liquid or gas (b.p. near room temperature) |
| Density | 1.14 g/cm3 (20 °C) |
| Melting point | -86 °C [1] |
| Boiling point | 23.5 °C |
| Dissolves | |
| Solubility | Soluble in benzene, toluene, ether |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Poisonous |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isocyanic acid is an inorganic compound with the formula HNCO, discovered in 1830 by Liebig and Wöhler.[2] This colourless substance is volatile and poisonous, with a boiling point of 23.5 °C. Isocyanic acid is the simplest stable chemical compound that contains carbon, hydrogen, nitrogen, and oxygen, the four most commonly found elements in organic chemistry and biology.
Preparation and reactions
Isocyanic acid can be made by protonation of the cyanate anion, such as from salts like potassium cyanate, by either gaseous hydrogen chloride or acids such as oxalic acid.[3]
- H+ + NCO- → HNCO
HNCO also can be made by the high-temperature thermal decomposition of cyanuric acid, a trimer.
- C3H3N3O3 → 3 HNCO
Isocyanic acid hydrolyses to carbon dioxide and ammonia:
- HNCO + H2O → CO2 + NH3
At sufficiently high concentrations, isocyanic acid oligomerizes to give cyanuric acid and cyamelide, a polymer. These species usually are easily separated from liquid- or gas-phase reaction products. Dilute solutions of isocyanic acid are stable in inert solvents, e.g. ether and chlorinated hydrocarbons.[4]
Isocyanic acid reacts with amines to give ureas (carbamides):
- HNCO + RNH2 → RNHC(O)NH2.
This reaction is called carbamylation.
HNCO adds across electron-rich double bonds, such as vinylethers, to give the corresponding isocyanates.
Isocyanic acid is also present in various forms of smoke, including smog and cigarette smoke. It was detected using mass spectrometry, and easily dissolves in water, posing a health risk to the lungs.[5]
Isomers: Cyanic acid and fulminic acid
Low-temperature photolysis of solids containing HNCO has been shown to make H-O-C≡N, known as cyanic acid or hydrogen cyanate; it is a tautomer of isocyanic acid.[6] Pure cyanic acid has not been isolated, and isocyanic acid is the predominant form in all solvents.[4] Note that sometimes information presented for cyanic acid in reference books is actually for isocyanic acid.
Cyanic and isocyanic acids are isomers of fulminic acid (H-C=N-O), an unstable compound.[7]
See also
References
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- ^ Liebig, J. (1830). "Untersuchungen über die Cyansäuren". Ann. Phys. 20 (11): 394. doi:10.1002/andp.18300961102.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Fischer, G. (2002). "Synthesis, Properties and Dimerization Study of Isocyanic Acid" (PDF). Z. Naturforschung. 57b (1): 19–25.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b A. S. Narula, K. Ramachandran “Isocyanic Acid” in Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley & Sons, New York. doi:10.1002/047084289X.ri072m Article Online Posting Date: April 15, 2001.
- ^ Preidt, Robert. "Chemical in Smoke May Pose Health Risk". MyOptumHealth. AccuWeather. Retrieved 14 September 2011.
- ^ Jacox, M.E. (1964). "Low-Temperature Infrared Study of Intermediates in the Photolysis of HNCO and DNCO". Journal of Chemical Physics. 40 (9): 2457–2460. doi:10.1063/1.1725546.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kurzer, Frederick (2000). "Fulminic Acid in the History of Organic Chemistry". Journal of Chemical Education. 77 (7): 851–857. doi:10.1021/ed077p851.
Further reading
- Handbook of Chemistry and Physics, 65th. Edition, CRC Press (1984)
External links
- Walter, Wolfgang (1997). Organic Chemistry: A Comprehensive Degree Text and Source Book. Chichester: Albion Publishing. p. 364. ISBN 9781898563372. Retrieved 2008-06-21.
- Cyanic acid from NIST Chemistry WebBook (accessed 2006-09-09)
