List of capsaicinoids
This is a list of capsaicinoids, a class of compound found in members of the capsicum family. They are the chemical responsible for making chili peppers hot. The heat intensity of capsaicinoids is measured in Scoville heat units (SCU) by the Scoville heat scale.[1]
List of capsaicinoids[edit]
| Structural formula | Name | Scoville heat units | Abbreviation | Reference |
|---|---|---|---|---|
 |
Resiniferatoxin | 16,000,000,000 | RTX | [2][3][4] |
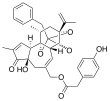 |
Tinyatoxin | 5,300,000,000 | TTX or TTN | [4] |

|
Phenylacetylrinvanil | 4,800,000,000 | IDN-5890 | [5][6][7] |

|
Capsaicin | 16,000,000 | CPS | [8] |
| Dihydrocapsaicin | 16,000,000 | DHC | [9] | |
| Nonivamide | 9,200,000 | PAVA | [10][9] | |

|
Nordihydrocapsaicin | 9,000,000 | NDHC | [9] |

|
Homocapsaicin | 8,600,000 | HC | [9] |
| Homodihydrocapsaicin | 8,600,000 | HDHC | [9] | |

|
Shogaol | 150,000 | 6-SGL | [9][11] |
 |
Piperine | 100,000 | PIP | [9][12] |

|
Gingerol | 80,000 | 6-G | [9][13] |

|
Capsiate | 16,000 | CAP | [14][15] |

|
Norcapsaicin | ~0[Footnote 1] | (NA) | [16][17][18] |
| Nornorcapsaicin | ~0[Footnote 1] | (NA) | [16][19][20] |
Footnotes[edit]
References[edit]
- ^ Nagy Z, Daood H, Ambrózy Z, Helyes L (2015). "Determination of Polyphenols, Capsaicinoids, and Vitamin C in New Hybrids of Chili Peppers". Journal of Analytical Methods in Chemistry. 2015: 102125. doi:10.1155/2015/102125. PMC 4606152. PMID 26495153.
- ^ Simon M. "This Chemical Is So Hot It Destroys Nerve Fibers—in a Good Way". Wired. ISSN 1059-1028. Retrieved 2023-08-25.
- ^ "Resiniferatoxin Is 1,000 Times Hotter Than Pure Hot Pepper Heat". ThoughtCo. Retrieved 2023-08-25.
- ^ a b Premkumar LS (November 2014). "Transient receptor potential channels as targets for phytochemicals". ACS Chemical Neuroscience. 5 (11): 1117–1130. doi:10.1021/cn500094a. PMC 4240255. PMID 24926802.
- ^ Appendino G, De Petrocellis L, Trevisani M, Minassi A, Daddario N, Moriello AS, et al. (February 2005). "Development of the first ultra-potent "capsaicinoid" agonist at transient receptor potential vanilloid type 1 (TRPV1) channels and its therapeutic potential". The Journal of Pharmacology and Experimental Therapeutics. 312 (2): 561–570. doi:10.1124/jpet.104.074864. PMID 15356216. S2CID 816699.
- ^ Luviano A, Aguiñiga-Sánchez I, Demare P, Tiburcio R, Ledesma-Martínez E, Santiago-Osorio E, Regla I (May 2014). "Antineoplastic activity of rinvanil and phenylacetylrinvanil in leukaemia cell lines". Oncology Letters. 7 (5): 1651–1656. doi:10.3892/ol.2014.1958. PMC 3997731. PMID 24765194.
- ^ Sánchez-Sánchez L, Alvarado-Sansininea JJ, Escobar ML, López-Muñoz H, Hernández-Vázquez JM, Monsalvo-Montiel I, et al. (July 2015). "Evaluation of the antitumour activity of Rinvanil and Phenylacetylrinvanil on the cervical cancer tumour cell lines HeLa, CaSKi and ViBo". European Journal of Pharmacology. 758: 129–136. doi:10.1016/j.ejphar.2015.04.003. PMID 25864613.
- ^ Hultquist M (2019-06-18). "The Scoville Scale". Chili Pepper Madness. Retrieved 2023-08-25.
- ^ a b c d e f g h "Dihydrocapsaicin - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2023-08-25.
- ^ Rohm B, Holik AK, Kretschy N, Somoza MM, Ley JP, Widder S, et al. (June 2015). "Nonivamide enhances miRNA let-7d expression and decreases adipogenesis PPARγ expression in 3T3-L1 cells". Journal of Cellular Biochemistry. 116 (6) (published 2015-04-10): 1153–1163. doi:10.1002/jcb.25052. PMC 4949678. PMID 25704235.
- ^ Kemkar KI, Sathiyanarayanan L, Sathiyanarayanan AR, Mahadik KA (2018-01-01). "6-Shogaol rich ginger oleoresin loaded mixed micelles enhances in vitro cytotoxicity on MCF-7 cells and in vivo anticancer activity against DAL cells". International Journal of Pharmacy and Pharmaceutical Sciences. 10: 160–168. doi:10.22159/ijpps.2018v10i1.23077. ISSN 0975-1491.
- ^ Azam S, Park JY, Kim IS, Choi DK (January 2022). "Piperine and Its Metabolite's Pharmacology in Neurodegenerative and Neurological Diseases". Biomedicines. 10 (1): 154. doi:10.3390/biomedicines10010154. PMC 8773267. PMID 35052833.
- ^ Mazyed EA, Badria FA, ElNaggar MH, El-Masry SM, Helmy SA (May 2022). "Development of Cyclodextrin-Functionalized Transethoniosomes of 6-Gingerol: Statistical Optimization, In Vitro Characterization and Assessment of Cytotoxic and Anti-Inflammatory Effects". Pharmaceutics. 14 (6): 1170. doi:10.3390/pharmaceutics14061170. PMC 9227240. PMID 35745746.
- ^ de Moura E, Silva VE, Cholewa JM, Jäger R, Zanchi NE, de Freitas MC, et al. (June 2021). "Chronic capsiate supplementation increases fat-free mass and upper body strength but not the inflammatory response to resistance exercise in young untrained men: a randomized, placebo-controlled and double-blind study". Journal of the International Society of Sports Nutrition. 18 (1): 50. doi:10.1186/s12970-021-00446-0. PMC 8218493. PMID 34154603.
- ^ Helmenstine A (2018-03-22). "Scoville Scale for Peppers and Other Hot Chemicals". Science Notes and Projects. Retrieved 2023-08-25.
- ^ a b Hamed M, Kalita D, Bartolo ME, Jayanty SS (September 2019). "Capsaicinoids, Polyphenols and Antioxidant Activities of Capsicum annuum: Comparative Study of the Effect of Ripening Stage and Cooking Methods". Antioxidants. 8 (9): 364. doi:10.3390/antiox8090364. PMC 6770197. PMID 31480665.
- ^ "Norcapsaicin". PubChem. U.S. National Library of Medicine. Retrieved 2023-08-25.
- ^ "Human Metabolome Database: Showing metabocard for Norcapsaicin (HMDB0036327)". hmdb.ca. Retrieved 2023-08-25.
- ^ "Human Metabolome Database: Showing metabocard for Dinorcapsaicin (HMDB0036325)". hmdb.ca. Retrieved 2023-08-25.
- ^ "Nornorcapsaicin". PubChem. U.S. National Library of Medicine. Retrieved 2023-08-25.

