Menshutkin reaction
The Menshutkin reaction in organic chemistry converts a tertiary amine to a quaternary ammonium salt by reaction with an alkyl halide:[1][2][3][4]

The reaction has been named after its discoverer, the Russian chemist Nikolai Menshutkin, who described the procedure in 1890. Depending on the source, his name (and the reaction named after him) is spelled as Menšutkin, Menshutkin or Menschutkin.
The reaction between amines and alkyl halogenides is hard to control, making alternative routes (for instance reductive amination) more attractive. However, when quaternary ammonium salts are the desired end product, this reaction becomes an interesting option. Yields are good and the reaction is easily performed. R1-R4 can but don't have to be identical. Some phase transfer catalysts (PTC) can be prepared according to the Menshutkin reaction, for instance the synthesis of triethyl benzyl ammonium chloride (TEBA) from triethylamine and benzyl chloride:

Reactions speed up with polar aprotic solvents and higher reaction temperatures. Leaving groups facilitate the reaction in the order chlorine < bromine < iodine.
Scope
In one particular macrocycle system the reaction rate is not only accelerated (150000 fold compared to quinuclidine) but the halide order is also changed:[5]
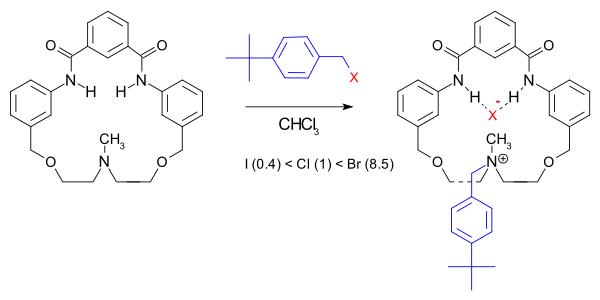
The rate-acceleration is attributed to increased transition state stabilization due to hydrogen bonding in the macrocyclic pocket.
References
- ^ N. Menschutkin. Beiträgen zur Kenntnis der Affinitätskoeffizienten der Alkylhaloide und der organischen Amine Z. Physik. Chem. 5 (1890) 589.
- ^ N Menschutkin. Über die Affinitätskoeffizienten der Alkylhaloide und der Amine Z. Physik. Chem. 6 (1890) 41.
- ^ M B Smith, J March. March's Advanced Organic Chemistry (Wiley, 2001) (ISBN 0-471-58589-0)
- ^ Lexikon bedeutender Chemiker (VEB Bibliographisches Institut Leipzig, 1989) (ISBN 3817110553
- ^ Dramatic Acceleration of the Menschutkin Reaction and Distortion of Halide Leaving-Group Order Keith J. Stanger, Jung-Jae Lee, and Bradley D. Smith J. Org. Chem. 2007, 72, 9663-9668 doi:10.1021/jo702090p
