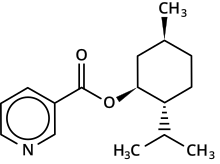
Menthyl nicotinate
| |

| |

| |
| Names | |
|---|---|
| IUPAC name
p-Menthan-3-yl pyridine-3-carboxylate
| |
| Systematic IUPAC name
5-Methyl-2-(propan-2-yl)cyclohexyl pyridine-3-carboxylate | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.049.975 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H23NO2 | |
| Molar mass | 261.365 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Odorless at room temperature, aromatic minty odor if warmed up |
| Density | 1.031 g/mL at 20 °C |
| Melting point | < −20 °C (−4 °F; 253 K) |
| Boiling point | 292.23 °C (558.01 °F; 565.38 K) |
| Insoluble | |
| Solubility | Soluble in polar oils, ethanol, organic solvents |
| log P | 5.09 @ 20 °C |
| Vapor pressure | 10 Pa @ 20 °C |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Eye irritant |
| GHS labelling: | |

| |
| Warning | |
| H319 | |
| P305+P351+P338 | |
| Flash point | 165 °C (329 °F; 438 K) @ 101 kPa |
| 354 °C (669 °F; 627 K) @ 102.3 kPa | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Menthyl nicotinate is an organic compound with the formula C16H23NO2. It is the ester of nicotinic acid (niacin, vitamin B3) and menthol. At room temperature, menthyl nicotinate is a colorless, odorless, viscous liquid.
Being a topical lipophilic niacin derivative, menthyl nicotinate is used in cosmetics and personal care products,[1] personal lubricants and intimate hygiene compositions.[2][3]
Menthyl nicotinate is rapidly absorbed through the stratum corneum and slowly hydrolyzed by skin esterase into niacin and menthol. Such time-dependent release of niacin and menthol, in an equimolar ratio, prevents the excessive niacin-flush effect that is usually observed with other nicotinates.[4]
Niacin is a precursor to coenzyme nicotinamide adenine dinucleotide (NAD), which is essential to all cellular processes involved in immune response and DNA-repairing of photodamaged skin cells.[5][6]
Niacin has also been used and tested for the purpose of enhancing detoxification by removing skin lipid-stored xenobiotics.[7][8][9][10]
In vitro testing has evidenced menthyl nicotinate's fast skin absorption kinetics and slow percutaneous delivery of niacin.[4]
Its antioxidant, detox, antipollution and protective efficacy against different kinds of damaging agents (UV radiation, oxidizing agents, urban particulates and cigarette smoke) has also been evaluated. Results indicate that menthyl nicotinate significantly enhances skin barrier function.[11]
References
[edit]- ^ "ECHA InfoCard".
- ^ US9,144,572 "Compositions and method for the stimulation of the female and male sexual response".
- ^ EP2881107B1 "Compositions and method for the stimulation of the female and male sexual response".
- ^ a b Segalla, G.; Giardina, S.; Bizzaro, G. (March–April 2019). "Menthyl Nicotinate – High rate of skin absorption and time-release delivery of Vitamin B3 (Niacin)" (PDF). Cosmetic Technology. 22 (2): 36–40.
- ^ Benavente, Claudia; Jacobson, Myron; Jacobson, Elaine (1 January 2009). "NAD in Skin: Therapeutic Approaches for Niacin". Current Pharmaceutical Design. 15 (1): 29–38. doi:10.2174/138161209787185760. PMID 19149600.
- ^ Jacobson, Elaine L.; Kim, Hyuntae; Kim, Moonsun; Williams, Joshua D.; Coyle, Donna L.; Coyle, W. Russell; Grove, Gary; Rizer, Ronald L.; Stratton, M. Suzanne; Jacobson, Myron K. (June 2007). "A topical lipophilic niacin derivative increases NAD, epidermal differentiation and barrier function in photodamaged skin". Experimental Dermatology. 16 (6): 490–499. doi:10.1111/j.1600-0625.2007.00553.x. PMID 17518989.
- ^ Prousky, Jonathan (January 2011). "Niacin for Detoxification: A Little-known Therapeutic Use". Journal of Orthomolecular Medicine. 26 (2): 85–92.
- ^ Schnare, David W.; Ben, Max; Shields, Megan G. (1984). "Body Burden Reductions of PCBs, PBBs and Chlorinated Pesticides in Human Subjects". Ambio. 13 (5/6): 378–380. JSTOR 4313080.
- ^ Dahlgren, James; Cecchini, Marie; Takhar, Harpreet; Paepke, Olaf (October 2007). "Persistent organic pollutants in 9/11 world trade center rescue workers: reduction following detoxification". Chemosphere. 69 (8): 1320–1325. doi:10.1016/j.chemosphere.2006.05.127. PMID 17234251.
- ^ Hoffer, Abram; Saul, Andrew W.; Foster, Harold D. (2015). Niacin: the real story. Basic Health. pp. 154–155. ISBN 9781591202752.
- ^ Segalla, G.; Giardina, S.; Bizzaro, G. (September–October 2018). "Nicomenthyl: transcutaneous niacin delivery and antipollution, detox, antioxidant efficacy" (PDF). Cosmetic Technology. 21 (5): 28–34.
