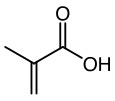
Methacrylic acid
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methylprop-2-enoic acid | |||
| Other names
Methacrylic acid
2-Methyl-2-propenoic acid α-Methacrylic acid 2-Methylacrylic acid 2-Methylpropenoic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | MAA | ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.096 | ||
| EC Number |
| ||
| MeSH | C008384 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H6O2 | |||
| Molar mass | 86.09 g/mol | ||
| Appearance | Colorless liquid or solid | ||
| Odor | Acrid, repulsive[1] | ||
| Density | 1.015 g/cm3 | ||
| Melting point | 14 to 15 °C (57 to 59 °F; 287 to 288 K) | ||
| Boiling point | 161 °C (322 °F; 434 K) | ||
| 9% (25 °C)[1] | |||
| Vapor pressure | 0.7 mmHg (20 °C)[1] | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 77.2 °C (171.0 °F; 350.3 K) | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[1] | ||
REL (Recommended)
|
TWA 20 ppm (70 mg/m3) [skin][1] | ||
IDLH (Immediate danger)
|
N.D.[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Methacrylic acid, abbreviated MAA, is an organic compound. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA) and poly(methyl methacrylate) (PMMA). MAA occurs naturally in small amounts in the oil of Roman chamomile.
Production
In the most common route, methacrylic acid is prepared from acetone cyanohydrin, which is converted to methacrylamide sulfate using sulfuric acid. This derivative in turn is hydrolyzed to methacrylic acid, or esterified to methyl methacrylate in one step. In the second route, isobutylene or tert-butanol are oxidized to methacrolein, then methacrylic acid. Methacrolein for this purpose can also be obtained from formaldehyde and ethylene. Isobutyric acid can also be dehydrogenated to methacrylic acid.[2]
It can also be prepared by decarboxylation of itaconic acid, citraconic acid, and mesaconic acid by decarboxylation. Such green precursors are not of commercial value. It is, however, obtained by boiling citra- or meso-brompyrotartaric acids with alkalis.
Uses
Methacrylic acid is used in some nail primers to help acrylic nails adhere to the nail plate.[3]
Reactions
Methacrylic acid was first obtained in the form of its ethyl ester by treating phosphorus pentachloride with oxyisobutyric ester (A synonym for beta-hydroxy-butyric acid or 3-hydroxybutyric acid[4]) .[5] When fused with an alkali, it forms propanoic acid. Sodium amalgam reduces it to isobutyric acid. A polymeric form of methacrylic acid was described in 1880.[6]

References
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0386". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Methacrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2002. doi:10.1002/14356007.a16_441. ISBN 978-3527306732.
{{cite encyclopedia}}: Unknown parameter|authors=ignored (help) - ^ "Products - Nail Care Products". www.fda.gov. U.S. Food and Drug Administration. 2018-03-06. Retrieved 2019-04-03.
- ^ https://www.vocabulary.com/dictionary/oxybutyric%20acid
- ^ Edward Frankland Annalen, 1865, 136, p. 12
- ^ F. Engelhorn et al. Ann., 1880, 200, p. 70.
- ^ Pham, Ha Q.; Marks, Maurice J. (2012). "Epoxy Resins". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_547.pub2.
External links
- [1] Methacrylic Acid in Europe.