Nafion

| |
| Identifiers | |
|---|---|
| ChemSpider |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C7HF13O5S . C2F4 | |
| Molar mass | See Article |
| Related compounds | |
Related compounds
|
Aciplex Flemion Dowew fumapem F |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nafion is a sulfonated tetrafluoroethylene based fluoropolymer-copolymer discovered in the late 1960s by Walther Grot of DuPont.[1] It is the first of a class of synthetic polymers with ionic properties which are called ionomers. Nafion's unique ionic properties are a result of incorporating perfluorovinyl ether groups terminated with sulfonate groups onto a tetrafluoroethylene (Teflon) backbone.[2][3] Nafion has received a considerable amount of attention as a proton conductor for proton exchange membrane (PEM) fuel cells because of its excellent thermal and mechanical stability.
The chemical basis of Nafion's superior conductive properties remain a focus of research. Protons on the SO3H (sulfonic acid) groups "hop" from one acid site to another. Pores allow movement of cations but the membranes do not conduct anions or electrons. Nafion can be manufactured with various cationic conductivities.
Nomenclature and molecular weight
Nafion can be produced as both a powder resin and a copolymer. It has various chemical configurations and thus several chemical names in the IUPAC system. Nafion-H, for example, includes the following systematic names:
- From Chemical Abstracts: ethanesulfonyl fluoride, 2-[1-[difluoro-[(trifluoroethenyl)oxy]methyl]-1,2,2,2-tetrafluoroethoxy]-1,1,2,2,-tetrafluoro-, with tetrafluoroethylene
- tetrafluoroethylene-perfluoro-3,6-dioxa-4-methyl-7-octenesulfonic acid copolymer
The molecular weight of Nafion is uncertain due to differences in processing and solution morphology.[2][3] The structure of a Nafion unit, shown at the top of the page, illustrates the variability of the material; for example, the most basic monomer contains chain variation between the ether groups (the z subscript). Conventional methods of determining molecular weight such as light scattering and gel permeation chromatography are not applicable because Nafion is insoluble, although the molecular weight has been estimated at 105–106 Da.[2][3] Instead, the equivalent weight (EW) and material thickness are used to describe most commercially available membranes. The EW is defined as the weight of Nafion (in terms of molecular mass) per sulfonic acid group.[3] For example, Nafion 117 indicates a material with 1100 g EW and 0.007 inches in thickness. In contrast to equivalent weight, conventional ion-exchange resins are usually described in terms of their ion exchange capacity (IEC), which is the multiplicative inverse or reciprocal of the equivalent weight, i.e., IEC = 1/EW.
Preparation
Nafion derivatives are first synthesized by the copolymerization of tetrafluoroethylene (TFE) (the monomer in Teflon) and a derivative of a perfluoro (alkyl vinyl ether) with sulfonyl acid fluoride. The latter reagent can be prepared by the pyrolysis of its respective oxide or carboxylic acid to give the olefinated structure.[4]
The resulting product is an -SO2F-containing thermoplastic that is extruded into films. Hot aqueous NaOH converts these sulfonyl fluoride (-SO2F) groups into sulfonate groups (-SO3−Na+). This form of Nafion, referred to as the neutral or salt form, is finally converted to the acid form containing the sulfonic acid (-SO3H) groups. Nafion can be cast into thin films by heating in aqueous alcohol at 250 °C in an autoclave. By this process, Nafion can be used to generate composite films, coat electrodes, or repair damaged membranes.[2]
Properties
The combination of the stable Teflon backbone with the acidic sulfonic groups gives Nafion its characteristics:[5]
- It is highly conductive to cations, making it suitable for many membrane applications.
- It resists chemical attack. According to DuPont, only alkali metals (particularly sodium) can degrade Nafion under normal temperatures and pressures.
- The Teflon backbone interlaced with the ionic sulfonate groups gives Nafion a high operating temperature, e.g. up to 190 °C, however, in membrane form, this is not possible due to the loss of water and mechanical strength.
- It is a superacid catalyst. The combination of fluorinated backbone, sulfonic acid groups, and the stabilizing effect of the polymer matrix make Nafion a very strong acid, with pKa ~ -6.[6] In this respect Nafion resembles the trifluoromethanesulfonic acid, CF3SO3H, although Nafion is a weaker acid by at least three orders of magnitude.
- It is selectively and highly permeable to water.
- Its proton conductivity up to 0.2 S/cm depending on temperature and hydration state[7]
- The solid phase and the aqueous phase of Nafion are both permeable to gases,[8][9] which is a drawback for energy conversion devices such as artificial leafs, fuel cells, and water electrolyzers.
Structure/morphology
The morphology of Nafion membranes is a matter of continuing study to allow for greater control of its properties. Other properties such as water management, hydration stability at high temperatures, electro-osmotic drag, as well as the mechanical, thermal, and oxidative stability, are affected by the Nafion structure.

The first model for Nafion, called the cluster-channel or cluster-network model, consisted of an equal distribution of sulfonate ion clusters (also described as 'inverted micelles'[3]) with a 40 Å (4 nm) diameter held within a continuous fluorocarbon lattice. Narrow channels about 10 Å (1 nm) in diameter interconnect the clusters, which explains the transport properties.[2][3][10]
The difficulty in determining the exact structure of Nafion stems from inconsistent solubility and crystalline structure among its various derivatives. Advanced morphological models have included a core-shell model where the ion-rich core is surrounded by an ion poor shell, a rod model where the sulfonic groups arrange into crystal-like rods, and a sandwich model where the polymer forms two layers whose sulfonic groups attract across an aqueous layer where transport occurs.[3] Consistency between the models include a network of ionic clusters; the models differ in the cluster geometry and distribution. Although no model has yet been determined fully correct, some scientists have demonstrated that as the membrane hydrates, Nafion's morphology transforms from the Cluster-Channel model to a rod-like model.[3]
A more recent water channel model[11] was proposed based on simulations of small-angle X-ray scattering data and solid state nuclear magnetic resonance studies. In this model, the sulfonic acid functional groups self-organize into arrays of hydrophilic water channels, each ~ 2.5 nm in diameter, through which small ions can be easily transported. Interspersed between the hydrophilic channels are hydrophobic polymer backbones which provide the observed mechanical stability.
Applications
Nafion's properties make it suitable for a broad range of applications. Nafion has found use in fuel cells, electrochemical devices, chlor-alkali production, metal-ion recovery, water electrolysis, plating, surface treatment of metals, batteries, sensors, Donnan dialysis cells, drug release, gas drying or humidifaction, and superacid catalysis for the production of fine chemicals.[2][3][5][12] Nafion is also often cited for theoretical potential (i.e., thus far untested) in a number of fields. With consideration of Nafion's wide functionality, only the most significant will be discussed below.
Chlor-alkali production cell membrane
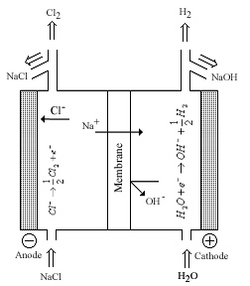
Chlorine and sodium/potassium hydroxide are among the most produced commodity chemicals in the world. Modern production methods produce Cl2 and NaOH/KOH from the electrolysis of brine using a Nafion membrane between half-cells. Before the use of Nafion, industries used mercury containing sodium amalgam to separate sodium metal from cells or asbestos diaphragms to allow for transfer of sodium ions between half cells; both technologies were developed in the latter half of the 19th century. The disadvantages of these systems is worker safety and environmental concerns associated with mercury and asbestos, although economical factors also played a part. Nafion was the direct result of the chlor-alkali industry addressing these concerns; Nafion could tolerate the high temperatures, high electrical currents, and corrosive environment of the electrolytic cells.[2][3][5]
The figure to the right shows a chlor-alkali cell where Nafion functions as a membrane between half cells. The membrane allows sodium ions to transfer from one cell to the other with minimal electrical resistance. The membrane was also reinforced with additional membranes to prevent gas product mixing and minimize back transfer of Cl− and −OH ions.[2] anode side positive and cathode side negative terminal is applied (shown wrong in diagram)
Proton exchange membrane (PEM) for fuel cells
Although fuel cells have been used since the 1960s as power supplies for satellites, recently they have received renewed attention for their potential to efficiently produce clean energy from hydrogen. Nafion was found effective as a membrane for proton exchange membrane (PEM) fuel cells by permitting hydrogen ion transport while preventing electron conduction. Solid Polymer Electrolytes, which are made by connecting or depositing electrodes (usually noble metal) to both sides of the membrane, conduct the electrons through an energy requiring process and rejoin the hydrogen ions to react with oxygen and produce water.[2] Fuel cells are expected to find strong use in the transportation industry.
Superacid catalyst for fine chemical production
Nafion, as a superacid, has potential as a catalyst for organic synthesis. Studies have demonstrated catalytic properties in alkylation, isomerization, oligomerization, acylation, ketalization, esterification, hydrolysis of sugars and ethers, and oxidation. New applications are constantly being discovered.[12] These processes, however, have not yet found strong commercial use. Several examples are shown below:
Alkylation with alkyl halides
Nafion-H gives efficient conversion whereas the alternative method, which employs Friedel-Crafts synthesis, can promote polyalkylation:[13]
Acylation
The amount of Nafion-H needed to catalyze the acylation of benzene with aroyl chloride is 10–30% less than the Friedel-Crafts catalyst:[13]
Catalysis of protection groups
Nafion-H increases reaction rates of protection via dihydropyran or o-trialkylsilation of alcohols, phenol, and carboxylic acids.[12]
Isomerization
Nafion can catalyze a 1,2-hydride shift.[12]
It is possible to immobilize enzymes within the Nafion by enlarging pores with lipophilic salts. Nafion maintains a structure and pH to provide a stable environment for the enzymes. Applications include catalytic oxidation of adenine dinucleotides.[12]
Sensors
Nafion has found use in the production of sensors, which with application in ion-selective, metallized, optical, and biosensors. What makes Nafion especially interesting is its demonstration in biocompatibility. Nafion has been shown to be stable in cell cultures as well as the human body, and there is considerable research towards the production of higher sensitivity glucose sensors.[2]
Modified Nafion for PEM fuel cells
Normal Nafion will dehydrate (thus lose proton conductivity) when temperature is above ~80 °C. This limitation troubles the design of fuel cells, because higher temperatures are desirable for a better efficiency and CO tolerance of the platinum catalyst. Silica and zirconium phosphate can be incorporated into Nafion water channels through in situ chemical reactions to increase the working temperature to above 100 °C.
References
- ^ Church, Steven (January 6, 2006). "Del. firm installs fuel cell". The News Journal. p. B7.
- ^ a b c d e f g h i j Heitner-Wirguin, C. (1996). "Recent advances in perfluorinated ionomer membranes: structure, properties and applications". Journal of Membrane Science. 120: 1–33. doi:10.1016/0376-7388(96)00155-X.
- ^ a b c d e f g h i j Mauritz, K. A., Moore, R. B.; Moore (2004). "State of Understanding of Nafion". Chemical Reviews. 104 (10): 4535–4585. doi:10.1021/cr0207123. PMID 15669162.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Connolly, D.J.; Longwood; Gresham, W. F. (1966). "Fluorocarbon Vinyl Ether Polymers". U.S. patent 3,282,875.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ a b c Perma Pure LLC (2004). "Nafion: Physical and Chemical Properties". Technical Notes and Articles. Archived from the original on September 28, 2013.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Schuster, M., Ise, M., Fuchs, A., Kreuer, K.D., Maier, J. (2005). Proton and Water Transport in Nano-separated Polymer Membranes (PDF). Germany: Max-Planck-Institut für Festkörperforschung.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "Journal of The Electrochemical Society — Error". doi:10.1149/1.1836625. Retrieved 2015-11-04.
- ^ Schalenbach, Maximilian; Hoefner, Tobias; Paciok, Paul; Carmo, Marcelo; Lueke, Wiebke; Stolten, Detlef (2015-10-28). "Gas Permeation through Nafion. Part 1: Measurements". The Journal of Physical Chemistry C. 119: 25145–25155. doi:10.1021/acs.jpcc.5b04155.
- ^ Schalenbach, Maximilian; Hoeh, Michael A.; Gostick, Jeff T.; Lueke, Wiebke; Stolten, Detlef (2015-10-14). "Gas Permeation through Nafion. Part 2: Resistor Network Model". The Journal of Physical Chemistry C. 119: 25156–25169. doi:10.1021/acs.jpcc.5b04157.
- ^ Gierke, T. D.; Munn, G. E.; Wilson, F. C. (1981). "The morphology in nafion perfluorinated membrane products, as determined by wide- and small-angle x-ray studies". Journal of Polymer Science: Polymer Physics Edition. 19 (11): 1687–1704. doi:10.1002/pol.1981.180191103.
- ^ Schmidt-Rohr, K.; Chen, Q. (2007). "Parallel cylindrical water nanochannels in Nafion fuel-cell membranes". Nature Materials. 7: 75–83. doi:10.1038/nmat2074. PMID 18066069.
- ^ a b c d e Gelbard, Georges (2005). "Organic Synthesis by Catalysis with Ion-Exchange Resins". Industrial & Engineering Chemistry Research. 44 (23): 8468–8498. doi:10.1021/ie0580405.
- ^ a b El-Kattan, Y.; McAtee, J.; Nafion-H. (2001) "Nafion-H". In Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, ISBN 978-0-470-01754-8.




