Nierenstein reaction
The Nierenstein reaction is an organic reaction describing the conversion of an acid chloride into an haloketone with diazomethane.[1][2] It is an insertion reaction in that the methylene from the diazomethane is inserted into the carbon-chlorine bond of the acid chloride.

Reaction mechanism
Like the related Arndt-Eistert reaction, this reactions proceeds through a diazoketone intermediate (5). The loss of nitrogen gives the desired haloketone (2).
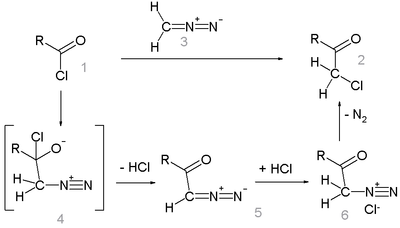
The synthesis of benzyl chloromethyl ketone from phenylacetyl chloride [3] in fact requires the addition of HCl gas to the diazoketone intermediate for it to succeed. The unassisted reaction failed.
Scope
One original 1924 Nierenstein reaction:[4]

and a reaction starting from benzoyl bromide going haywire with formation of the dioxane dimer:[5]

References
- ^ Clibbens, D.; Nierenstein, M. (1915). "The action of diazomethane on some aromatic acyl chlorides". J. Chem. Soc. 107: 1491. doi:10.1039/CT9150701491.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bachman, W. E.; Struve, W. S. (1942). Org. React. 1: 38.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) (Review) - ^ McPhee, W. D; Klingsberg, E. Organic Syntheses, Coll. Vol. 3, p.119 (1955); Vol. 26, p.13 (1946). (Article)
- ^ M. Nierenstein, D. G. Wang, and J. C. Warr (1924). "The Action of Diazomethane on some Aromatic Acyl Chlorides II. Synthesis of Fisetol". J. Am. Chem. Soc. 46 (11): 2551–2555. doi:10.1021/ja01676a028.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ H. H. Lewis, M. Nierenstein, and Enid M. Rich (1925). "The Action of Diazomethane on some Aromatic Acyl Chlorides III. The Mechanism of the Reaction". J. Am. Chem. Soc. 47 (6): 1728–1732. doi:10.1021/ja01683a036.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
