Nitrogen oxide: Difference between revisions
m Reverting edits identified as vandalism. (settings/false-positives) |
No edit summary |
||
| Line 1: | Line 1: | ||
| ⚫ | |||
{{TOCright}} |
|||
| ⚫ | |||
* [[Nitric oxide]] (NO), nitrogen(II) oxide |
* [[Nitric oxide]] (NO), nitrogen(II) oxide |
||
Revision as of 20:07, 28 September 2010
this is my science essayNitrogen oxide can refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
- Nitric oxide (NO), nitrogen(II) oxide
- Nitrogen dioxide (NO2), nitrogen(IV) oxide
- Nitrous oxide (N2O), nitrogen(I) oxide
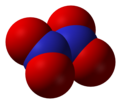
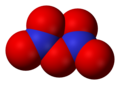
- Nitrate radical (NO3), nitrogen(VI) oxide
- Dinitrogen trioxide (N2O3), nitrogen(II,IV) oxide
- Dinitrogen tetroxide (N2O4), nitrogen(IV) oxide
- Dinitrogen pentoxide (N2O5), nitrogen(V) oxide
In atmospheric chemistry and air pollution and related fields, nitrogen oxides refers specifically to NOx (NO and NO2).[1][2]
Only the first three of these compounds can be isolated at room temperature. N2O3, N2O4, and N2O5 all decompose rapidly at room temperature. Nitrate radical is very reactive.
N2O is stable and rather unreactive at room temperature, while NO and NO2 are quite reactive but nevertheless quite stable when isolated.
-
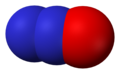
Nitric oxide, NO -
Nitrogen dioxide, NO2 -
Nitrous oxide, N2O -
Dinitrogen trioxide, N2O3 -
Dinitrogen tetroxide, N2O4 -
Dinitrogen pentoxide, N2O5
NOx
NOx (often written NOx) refers to NO and NO2. They are produced during combustion, especially at high temperature. These two chemicals are important trace species in Earth's atmosphere. In the troposphere, during daylight, NO reacts with partly oxidized organic species (or the peroxy radical) to form NO2, which is then photolyzed by sunlight to reform NO:
- NO + CH3O2 → NO2 + CH3O
- NO2 + sunlight → NO + O
The oxygen atom formed in the second reaction then goes on to form ozone; this series of reactions is the main source of tropospheric ozone. CH3O2 is just one example of many partly oxidized organic molecules that can react with NO to form NO2.
These reactions are rather fast so NO and NO2 cycle, but the sum of their concentration ([NO] + [NO2]) tends to remain fairly constant. Because of this cycling, it is convenient to think of the two chemicals as a group; hence the term NOx.
In addition to acting as a main precursor for tropospheric ozone, NOx is also harmful to human health in its own right.
Derivatives
Oxidized (cationic) and reduced (anionic) derivatives of many of these oxides exist: nitrite (NO2-), nitrate (NO3-), nitronium (NO2+), and nitrosonium (NO+). NO2 is intermediate between nitrite and nitronium:
- NO2+ + e− → NO2
- NO2 + e− → NO2−
See also
References
- ^ United States Clean Air Act, 42 U.S.C. § 7602
- ^ Seinfeld, John H.; Pandis, Spyros N., Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, Wiley-Interscience, ISBN 0471178160