Norsteroid
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
Norsteroids (nor-, L. norma, from "normal" in chemistry, indicating carbon removal) are a structural class of steroids that have had an atom or atoms (typically carbon) removed, biosynthetically or synthetically, from positions of branching off of rings or side chains (e.g., removal of methyl groups), or from within rings of the steroid ring system.[1][2] For instance, 19-norsteroids (e.g., 19-norprogesterone) constitute an important class of natural and synthetic steroids derived by removal of the methyl group of the natural product progesterone; the equivalent change between testosterone and 19-nortestosterone (nandrolone) is illustrated below.
Examples
Norsteroid examples include: norpregnene (from pregnene), desogestrel, ethylestrenol, etynodiol diacetate, ethinyl estradiol, gestrinone, levonorgestrel, norethisterone (norethindrone), norgestrel, norpregnatriene (from pregnatriene), quinestrol, 19-norprogesterone (from a progesterone), Nomegestrol acetate,[3][non-primary source needed] 19-nortestosterone (from a testosterone), and norethisterone acetate.[3][non-primary source needed]
 |

| |
| progesterone | 19-norprogesterone | |
 |
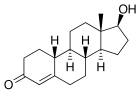
| |
| testosterone | nortestosterone (nandrolone) |
References
- ^ International Union of Pure and Applied Chemistry (IUPAC), 1999, "RF-4.1 Removal of Skeletal Atoms," in "RF-4. Skeletal Modifications" in Revised Section F: Natural Products and Related Compounds (IUPAC Recommendations 1999), see [1], accessed 20 May 2014. See also IUPAC, 1976, "Nomenclature of Organic Chemistry: Section F - Natural Products and Related Compounds, Recommendations 1976", IUPAC Information Bulletin Appendices on Tentative Nomenclature, Symbols, Units, and Standards, No. 53, December, 1976, also in Eur. J. Biochem. 1978, 86, 1-8.
- ^ Norsteroids at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ^ a b Couzinet B, Young J, Brailly S, Chanson P, Thomas JL, Schaison G (December 1996). "The antigonadotropic activity of progestins (19-nortestosterone and 19-norprogesterone derivatives) is not mediated through the androgen receptor". J. Clin. Endocrinol. Metab. 81 (12): 4218–23. doi:10.1210/jc.81.12.4218. PMID 8954018.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- Bricout V, Wright F (2004). "Update on nandrolone and norsteroids: how endogenous or xenobiotic are these substances?". Eur J Appl Physiol. 92 (1–2): 1–12. doi:10.1007/s00421-004-1051-3. PMID 15042372.
