Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, C
6H
5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.
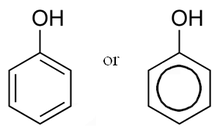
Phenols are synthesized industrially and produced by plants and microorganisms, with variation between and within species.[1]
Properties
Acidity
Phenols have distinct properties and are generally distinguished from other alcohols. They have higher acidities. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Loss of a hydrogen cation (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.
Oxidation
Phenols are reactive species toward oxidation. Oxidative cleavage, for instance cleavage of 1,2-dihydroxybenzene to the monomethylester of 2,4 hexadienedioic acid with oxygen, copper chloride in pyridine[2]
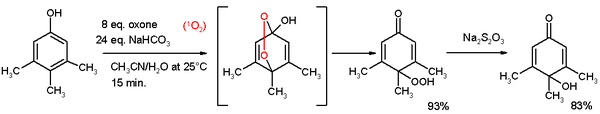
- Oxidative de-aromatization to quinones also known as the Teuber reaction. Oxidizing reagents are Fremy's salt[3] and oxone.[4] In reaction depicted below 3,4,5-trimethylphenol reacts with singlet oxygen generated from oxone/sodium carbonate in an acetonitrile/water mixture to a para-peroxyquinole. This hydroperoxide is reduced to the quinole with sodium thiosulfate.
- Phenols are oxidized to hydroquinones in the Elbs persulfate oxidation
Electrophilic aromatic substitution
Phenols are highly susceptible to Electrophilic aromatic substitutions. Illustrative of a large-scale electrophilic aromatic substitution is the production of bisphenol A, which is produced on a scale 1 million tons. This compound is synthesized by the condensation of acetone.[5]
Other reactions
Phenols undergo esterfication. Phenol esters are active esters, being prone to hydrolysis.
Reaction of naphtols and hydrazines and sodium bisulfite in the Bucherer carbazole synthesis
Synthesis

Several laboratory methods for the synthesis of phenols:
- by an ester rearrangement in the Fries rearrangement
- by a rearrangement of N-phenylhydroxylamines in the Bamberger rearrangement
- by dealkylation of phenolic ethers
- by reduction of quinones
- by replacement of an aromatic amine by an hydroxyl group with water and sodium bisulfide in the Bucherer reaction
- by thermal decomposition of aryl diazonium salts, the salts are converted to phenol
- by oligomerization with formaldehyde + base catalyzed reaction with epichlorohydrin to epoxy resin components
- by reaction with acetone/ketones to e.g. bisphenol A, an important monomer for resins, e.g. polycarbonate (PC), epoxy resins
- by the oxidation of aryl silanes—an aromatic variation of the Fleming-Tamao oxidation [7]
- by the addition of benzene and propene in H
3PO
4 to form cumene then O
2 is added with H
2SO
4 to form phenol (Hock process)
Classification
There are various classification schemes.[8]: 2 A commonly used scheme is based on the number of carbons and was devised by Jeffrey Harborne and Simmonds in 1964 and published in 1980:[8]: 2 [9]

| Phenol | the parent compound, used as a disinfectant and for chemical synthesis |
| Bisphenol A | and other bisphenols produced from ketones and phenol / cresol |
| BHT | (butylated hydroxytoluene) - a fat-soluble antioxidant and food additive |
| 4-Nonylphenol | a breakdown product of detergents and nonoxynol-9 |
| Orthophenyl phenol | a fungicide used for waxing citrus fruits |
| Picric acid | (trinitrophenol) - an explosive material |
| Phenolphthalein | pH indicator |
| Xylenol | used in antiseptics & disinfectants |
Drugs, present and past
| Diethylstilbestrol | a synthetic estrogen with a stilbene structure; no longer marketed |
| L-DOPA | a dopamine prodrug used to treat Parkinson's disease |
| Propofol | a short-acting intravenous anesthetic agent |

The majority of these compounds are soluble molecules but the smaller molecules can be volatile.[citation needed]
Phenols chemically interact with many other substances.[citation needed] Stacking, a chemical property of molecules with aromaticity, is seen occurring between phenolic molecules.[citation needed] When studied in mass spectrometry, phenols easily form adduct ions with halogens.[citation needed] They can also interact with the food matrices or with different forms of silica (mesoporous silica, fumed silica[10] or silica-based sol gels[11]).
References
- ^ Hättenschwiler, Stephan; Vitousek, Peter M. (2000). "The role of polyphenols in terrestrial ecosystem nutrient cycling". Trends in Ecology & Evolution. 15 (6): 238–243. doi:10.1016/S0169-5347(00)01861-9.
- ^ 2,4-Hexadienedioic acid, monomethyl ester, (Z,Z)- Organic Syntheses, Coll. Vol. 8, p.490 (1993); Vol. 66, p.180 (1988) Article
- ^ "2,5-Cyclohexadiene-1,4-dione, 2,3,5-trimethyl". Organic Syntheses, Coll. 6: 1010. 1988.; Vol. 52, p.83 (1972) Abstract.
- ^ Carreño, M. Carmen; González-López, Marcos; Urbano, Antonio (2006). "Oxidative De-aromatization of para-Alkyl Phenols into para-Peroxyquinols and para-Quinols Mediated by Oxone as a Source of Singlet Oxygen". Angewandte Chemie International Edition. 45 (17): 2737–2741. doi:10.1002/anie.200504605. PMID 16548026.
- ^ Fiege H; Voges H-W; Hamamoto T; Umemura S; Iwata T; Miki H; Fujita Y; Buysch H-J; Garbe D; Paulus W (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313. ISBN 978-3527306732.
- ^ mostami, Ros (2017). "Facile preparation of Ʌ-shaped building blocks: Hünlich-base derivatization". Synlett. 28: 1641–1645. doi:10.1055/s-0036-1588180.
- ^ Bracegirdle, Sonia; Anderson, Edward A. (2010). "Arylsilane oxidation—new routes to hydroxylated aromatics". Chem. Comm. 46 (20): 3454–6. doi:10.1039/b924135c. PMID 20582346.
- ^ a b Wilfred Vermerris and Ralph Nicholson. Phenolic Compound Biochemistry Springer, 2008
- ^ Harborne, J. B. (1980). "Plant phenolics". In Bell, E. A.; Charlwood, B. V. (eds.). Encyclopedia of Plant Physiology, volume 8 Secondary Plant Products. Berlin Heidelberg New York: Springer-Verlag. pp. 329–395.
- ^ Kulik, T. V.; Lipkovska, N. A.; Barvinchenko, V. N.; Palyanytsya, B. B.; Kazakova, O. A.; Dovbiy, O. A.; Pogorelyi, V. K. (2009). "Interactions between bioactive ferulic acid and fumed silica by UV–vis spectroscopy, FT-IR, TPD MS investigation and quantum chemical methods". Journal of Colloid and Interface Science. 339 (1): 60–8. Bibcode:2009JCIS..339...60K. doi:10.1016/j.jcis.2009.07.055. PMID 19691966.
- ^ Lacatusu, Ioana; Badea, Nicoleta; Nita, Rodica; Murariu, Alina; Miculescu, Florin; Iosub, Ion; Meghea, Aurelia (2010). "Encapsulation of fluorescence vegetable extracts within a templated sol–gel matrix". Optical Materials. 32 (6): 711–718. Bibcode:2010OptMa..32..711L. doi:10.1016/j.optmat.2009.09.001.